10a motor system locomotion
- 2. Control of voluntary movement Idea Association cortex Premotor + Motor cortex Basal Ganglia Lateral cerebellum Movement Intermediate Cerebellum Execution Planning
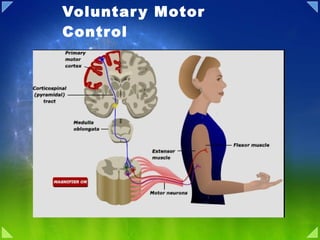
- 3. Organization of Motor Nervous System
- 5. Spinal Cord: Grey matter
- 6. Motor Unit
- 11. Mechanism of Muscle contraction
- 12. Types of Muscle Contraction
- 13. Muscle Types
- 14. The Regulation of Muscle force
- 15. Muscle Spindle
- 16. Muscle Spindle: Stretch Receptor
- 17. Stretch Reflex
- 22. Feedback Inhibition: Golgi Tendon Organ
- 24. Spinal Animal
- 25. The Motor Pattern for Stepping in Mammals
- 26. Cat Walk
- 27. Cat hind limb activity during stepping
- 30. Sensory Feedback for walking
- 31. Locomotor Center in Cat
- 32. Mesencephalic locomotor center stimulation in Cat
- 34. Human Locomotion
Editor's Notes
- Summary Four distinct but highly interactive motor subsystems—local circuits in the spinal cord and brainstem, descending upper motor neuron pathways that controlthese circuits, the basal ganglia, and the cerebellum—all make essential contributions to motor control. Alpha motor neurons located in the spinal cord and in the cranial nerve nuclei in the brainstem directly link the nervous system and muscles, with each motor neuron and its associated muscle fibers constituting a functional entity called the motor unit. Motor units vary in size, amount of tension produced, speed of contraction, and degree of fatigability. Graded increases in muscle tension are mediated by both the orderly recruitment of different types of motor units and an increase in motor neuron firing frequency. Local circuitry involving sensory inputs, local circuit neurons, and α and γ motor neurons are especially important in the reflexive control of muscle activity. The stretch reflex is a monosynaptic circuit with connections between sensory fibers arising from muscle spindles and the α motor neurons that innervate the same or synergistic muscles. Gamma motor neurons regulate the gain of the stretch reflex by adjusting the level of tension in the intrafusal muscle fibers of the muscle spindle. This mechanism sets the baseline level of activity in α motor neurons and helps to regulate muscle length and tone. Other reflex circuits provide feedback control of muscle tension and mediate essential functions such as the rapid withdrawal of limbs from painful stimuli. Much of the spatial coordination and timing of muscle activation required for complex rhythmic movements such as locomotion are provided by specialized local circuits called central pattern generators. Because of their essential role in all of these circuits, damage to lower motor neurons leads to paralysis of the associated muscle and to other changes, including the loss of reflex activity, the loss of muscle tone, and eventually muscle atrophy.
- Nevertheless, there is considerable evidence for the general motor control scheme shown in Figure 12-1 . Commands for voluntary movement originate in cortical association areas. The movements are planned in the cortex as well as in the basal ganglia and the lateral portions of the cerebellar hemispheres, as indicated by increased electrical activity before the movement. The basal ganglia and cerebellum both funnel information to the premotor and motor cortex by way of the thalamus. Motor commands from the motor cortex are relayed in large part via the corticospinal tracts to the spinal cord and the corresponding corticobulbar tracts to motor neurons in the brain stem. However, collaterals from these pathways and a few direct connections from the motor cortex end on brain stem nuclei, which also project to motor neurons in the brain stem and spinal cord. These pathways can also mediate voluntary movement. Movement sets up alterations in sensory input from the special senses and from muscles, tendons, joints, and the skin. This feedback information, which adjusts and smoothes movement, is relayed directly to the motor cortex and to the spinocerebellum. The spinocerebellum projects in turn to the brain stem. The main brain stem pathways that are concerned with posture and coordination are the rubrospinal, reticulospinal, tectospinal, and vestibulospinal tracts and corresponding projections to motor neurons in the brain stem.
- Figure 16.1. Overall organization of neural structures involved in the control of movement. Four systems—local spinal cord and brainstem circuits, descending modulatory pathways, the basal ganglia, and the cerebellum—make essential and distinct contributions to motor control. Lower Motor Neuron Circuits and Motor Control Overview Skeletal (striated) muscle contraction is initiated by “lower” motor neurons in the spinal cord and brainstem. The cell bodies of the lower neurons are located in the ventral horn of the spinal cord gray matter and in the motor nuclei of the cranial nerves in the brainstem. These neurons (also called α motor neurons) send axons directly to skeletal muscles via the ventral roots and spinal peripheral nerves, or via cranial nerves in the case of the brainstem nuclei. The spatial and temporal patterns of activation of lower motor neurons are determined primarily by local circuits located within the spinal cord and brainstem. Descending pathways comprising the axons of “upper” motor neurons modulate the activity of lower motor neurons by influencing this local circuitry. The cell bodies of upper motor neurons are located either in the cortex or in brainstem centers, such as the vestibular nucleus, the superior colliculus, and the reticular formation. The axons of the upper motor neurons typically contact the local circuit neurons in the brainstem and spinal cord, which, via relatively short axons, contact in turn the appropriate combinations of lower motor neurons. The local circuit neurons also receive direct input from sensory neurons, thus mediating important sensory motor reflexes that operate at the level of the brainstem and spinal cord. Lower motor neurons, therefore, are the final common pathway for transmitting neural information from a variety of sources to the skeletal muscles. Neural Centers Responsible for Movement The neural circuits responsible for the control of movement can be divided into four distinct but highly interactive subsystems, each of which makes a unique contribution to motor control ( Figure 16.1 ). The first of these subsystems is the local circuitry within the gray matter of the spinal cord and the analogous circuitry in the brainstem. The relevant cells include the lower motor neurons (which send their axons out of the brainstem and spinal cord to innervate the skeletal muscles of the head and body, respectively) and the local circuit neurons (which are the major source of synaptic input to the lower motor neurons). All commands for movement, whether reflexive or voluntary, are ultimately conveyed to the muscles by the activity of the lower motor neurons; thus they comprise, in the words of the great British neurophysiologist Charles Sherrington, the “final common path” for movement. The local circuit neurons receive sensory inputs as well as descending projections from higher centers. Thus, the circuits they form provide much of the coordination between different muscle groups that is essential for organized movement. Even after the spinal cord is disconnected from the brain in an experimental animal such as a cat, appropriate stimulation of local spinal circuits elicits involuntary but highly coordinated movements of the four limbs that resemble walking. The second motor subsystem consists of neurons whose cell bodies lie in the brainstem or cerebral cortex. The axons of these higher-order or upper motorneurons descend to synapse with the local circuit neurons or, more rarely, with the lower motor neurons directly. The upper motor neuron pathways that arise in the cortex are essential for the initiation of voluntary movements and for complex temporal sequences of movement. In particular, descending projections from cortical areas in the frontal lobe, including Brodmann's area 4 (the primary motor cortex ), the lateral part of area 6 (the lateral premotor cortex ), and the medial part of area 6 (the medial premotor cortex ) are essential for planning, initiating, and directing temporal sequences of voluntary movements. Upper motor neurons originating in the brainstem are responsible for regulating muscle tone and for orienting the eyes, head, and body with respect to vestibular, somatic, auditory, and visual sensory information. Their contributions are thus critical for basic navigational movements of the body, and in the control of posture. The third and fourth subsystems are structures (or groups of structures) that have no direct access to either the local circuit neurons or the lower motorneurons; instead, they control movement by regulating the activity of the upper motor neurons. The third and larger of these subsystems, the cerebellum , is located on the dorsal surface of the pons (see Chapter 1 ). The cerebellum acts via its efferent pathways to the upper motor neurons as a servomechanism, detecting the difference, or “motor error,” between an intended movement and the movement actually performed (see Chapter 19 ). The cerebellum uses this information about discrepancies to mediate both real-time and long-term reductions in these motor errors (the latter being a form of motor learning). As might be expected from this account, patients with cerebellar damage exhibit persistent errors in movement. The fourth subsystem, embedded in the depths of the forebrain, consists of a group of structures collectively referred to as the basal ganglia (see Chapter 1 ). The basal ganglia suppress unwanted movements and prepare (or “prime”) upper motor neuron circuits for the initiation of movements. The problems associated with disorders of basal ganglia, such as Parkinson's disease and Huntington's disease, attest to the importance of this complex in the initiation of voluntary movements (see Chapter 18 ). Despite much effort, the sequence of events that leads from thought to movement is still poorly understood. The picture is clearest, however, at the level of control of the muscles themselves. It therefore makes sense to begin an account of motor behavior by considering the anatomical and physiological relationships between lower motor neurons and the muscle fibers they innervate The Motor Systems Are Organized Hierarchicaly The Spinal Cord, Brain Stem, and Forebrain Contain Successively More Complex Motor Circuits The motor systems can perform so many different motor tasks—reflex, rhythmic, and voluntary—with speed and accuracy because of two features of their functional organization. First, the processing of sensory inputs and commands to motor neurons and muscles is distributed in hierarchically interconnected areas of the spinal cord, brain stem, and forebrain. Each level has circuits that can, through their input and output connections, organize or regulate complex motor responses. Second, sensory information relating to movement is processed in different systems that operate in parallel. The hierarchical organization of the motor systems is illustrated in Figure 33-12. The spinal cord is the lowest level of this hierarchical organization. It contains the neuronal circuits that mediate a variety of reflexes and rhythmic automatisms such as locomotion and scratching. Similar circuits governing reflex movements of the face and mouth are located in the brain stem. The simplest neural circuit is monosynaptic; it includes only the primary sensory neuron and the motor neuron. However, most reflexes are mediated by polysynaptic circuits, where one or more interneurons are interposed between the primary sensory neuron and the motor neuron. Interneurons and motor neurons also receive input from axons descending from higher centers. These supraspinal signals can modify reflex responses to peripheral stimuli by facilitating or inhibiting different populations of interneurons. They also coordinate motor actions through these interneurons. For example, when we flex a joint the descending commands that drive the flexor muscle also inhibit the opposing extensor muscle through the same inhibitory interneuron that is activated during the stretch reflex. Nevertheless, all motor commands eventually converge on motor neurons, whose axons exit the spinal cord or brain stem to innervate skeletal muscles. Thus in Sherrington's words, motor neurons are the “final common pathway” for all motor action. The next level of the motor hierarchy is in the brain stem. Two systems of brain stem neurons, the medial and lateral, receive input from the cerebral cortex and subcortical nuclei and project to the spinal cord. The medial descending systems of the brain stem contribute to the control of posture by integrating visual, vestibular, and somatosensory information. The lateral descending systems control more distal limb muscles and are thus important for goal-directed movements, especially of the arm and hand. Other brain stem circuits control movements of the eyes and head. The cortex is the highest level of motor control. The primary motor cortex and several premotor areas project directly to the spinal cord through the corticospinal tract and also regulate motor tracts that originate in the brain stem. The premotor areas are important for coordinating and planning complex sequences of movement. They receive information from the posterior parietal and prefrontal association cortices (see Chapter 19) and project to the primary motor cortex as well as to the spinal cord. The variety of reflex circuits in the spinal cord and brain stem simplifies the instructions the cortex must send to lower levels. By facilitating some circuits and inhibiting others, higher levels can let sensory inputs at lower levels govern the temporal details of an evolving movement. The timing of activation of agonists and antagonist muscles is intrinsic to the spinal circuit and thus the descending signals themselves need not be timed as precisely. The patterns of coordination in spinal circuits are relatively stereotyped. A cat with its cervical cord transected can, if provided with body support, walk on a moving treadmill and bring its paw around an obstacle after hitting it. But the spinal cat cannot lift its forelimb before impact with an obstacle, as an intact animal does, because this movement requires control of the limbs using visual information. This anticipatory control, in turn, requires intervention by the motor cortex to suppress the oscillatory circuit that coordinates normal stepping. The Cerebellum and Basal Ganglia Influence Cortical and Brain Stem Motor Systems In addition to the three hierarchical levels—spinal cord, brain stem, and cortex—two other parts of the brain also regulate the planning and execution of movement. The cerebellum and basal ganglia provide feedback circuits that regulate cortical and brain stem motor areas: They receive inputs from various areas of cortex and project to motor areas of the cortex via the thalamus. The loop circuits of these two structures flow through separate regions of the thalamus and to different cortical areas. Likewise, the inputs to them from the cortex are also separate. The cerebellum and basal ganglia do not send significant output to the spinal cord, but they do act directly on motor neurons in the brain stem. The Motor Systems Are Organized Hierarchicaly The Spinal Cord, Brain Stem, and Forebrain Contain Successively More Complex Motor Circuits The motor systems can perform so many different motor tasks—reflex, rhythmic, and voluntary—with speed and accuracy because of two features of their functional organization. First, the processing of sensory inputs and commands to motor neurons and muscles is distributed in hierarchically interconnected areas of the spinal cord, brain stem, and forebrain. Each level has circuits that can, through their input and output connections, organize or regulate complex motor responses. Second, sensory information relating to movement is processed in different systems that operate in parallel. The hierarchical organization of the motor systems is illustrated in Figure 33-12. The spinal cord is the lowest level of this hierarchical organization. It contains the neuronal circuits that mediate a variety of reflexes and rhythmic automatisms such as locomotion and scratching. Similar circuits governing reflex movements of the face and mouth are located in the brain stem. The simplest neural circuit is monosynaptic; it includes only the primary sensory neuron and the motor neuron. However, most reflexes are mediated by polysynaptic circuits, where one or more interneurons are interposed between the primary sensory neuron and the motor neuron. Interneurons and motor neurons also receive input from axons descending from higher centers. These supraspinal signals can modify reflex responses to peripheral stimuli by facilitating or inhibiting different populations of interneurons. They also coordinate motor actions through these interneurons. For example, when we flex a joint the descending commands that drive the flexor muscle also inhibit the opposing extensor muscle through the same inhibitory interneuron that is activated during the stretch reflex. Nevertheless, all motor commands eventually converge on motor neurons, whose axons exit the spinal cord or brain stem to innervate skeletal muscles. Thus in Sherrington's words, motor neurons are the “final common pathway” for all motor action. The next level of the motor hierarchy is in the brain stem. Two systems of brain stem neurons, the medial and lateral, receive input from the cerebral cortex and subcortical nuclei and project to the spinal cord. The medial descending systems of the brain stem contribute to the control of posture by integrating visual, vestibular, and somatosensory information. The lateral descending systems control more distal limb muscles and are thus important for goal-directed movements, especially of the arm and hand. Other brain stem circuits control movements of the eyes and head. The cortex is the highest level of motor control. The primary motor cortex and several premotor areas project directly to the spinal cord through the corticospinal tract and also regulate motor tracts that originate in the brain stem. The premotor areas are important for coordinating and planning complex sequences of movement. They receive information from the posterior parietal and prefrontal association cortices (see Chapter 19) and project to the primary motor cortex as well as to the spinal cord. The variety of reflex circuits in the spinal cord and brain stem simplifies the instructions the cortex must send to lower levels. By facilitating some circuits and inhibiting others, higher levels can let sensory inputs at lower levels govern the temporal details of an evolving movement. The timing of activation of agonists and antagonist muscles is intrinsic to the spinal circuit and thus the descending signals themselves need not be timed as precisely. The patterns of coordination in spinal circuits are relatively stereotyped. A cat with its cervical cord transected can, if provided with body support, walk on a moving treadmill and bring its paw around an obstacle after hitting it. But the spinal cat cannot lift its forelimb before impact with an obstacle, as an intact animal does, because this movement requires control of the limbs using visual information. This anticipatory control, in turn, requires intervention by the motor cortex to suppress the oscillatory circuit that coordinates normal stepping. The Cerebellum and Basal Ganglia Influence Cortical and Brain Stem Motor Systems In addition to the three hierarchical levels—spinal cord, brain stem, and cortex—two other parts of the brain also regulate the planning and execution of movement. The cerebellum and basal ganglia provide feedback circuits that regulate cortical and brain stem motor areas: They receive inputs from various areas of cortex and project to motor areas of the cortex via the thalamus. The loop circuits of these two structures flow through separate regions of the thalamus and to different cortical areas. P.664 Likewise, the inputs to them from the cortex are also separate. The cerebellum and basal ganglia do not send significant output to the spinal cord, but they do act directly on motor neurons in the brain stem. Lesions of the Motor Pathways Produce Positive and Negative Signs The nineteenth-century neurologist John Hughlings Jackson, whose clinical insights were so informative for the early understanding of different regions of cortex (Chapter 19), was also the first to recognize that lesions of the nervous system result in both negative and positive signs. Negative signs reflect the loss of particular capacities normally controlled by the damaged system, for example loss of strength. Positive signs, also called release phenomena , are abnormal and stereotyped responses that are explained by the withdrawal of tonic inhibition from neuronal circuits mediating a behavior. When cerebral control of the brain stem is disconnected in the cat, ordinary head and neck movements produce postural reflexes that otherwise do not occur in the intact animal. In humans, lesions that interrupt the descending pathways from the cortex or brain stem produce weakness in voluntary movements (a negative sign) and, at the same time, increase muscle tone, a key feature of the clinical picture of spasticity. In this condition, as in decerebrate rigidity, stretch reflexes are abnormally active. Clinicians often must decide if a patient's weakness arises from a disease that affects systems descending from the cortex and brain stem to motor neurons or from a disease that directly affects the motor neurons or their axons. Although both conditions produce weakness by diminishing neural input to muscle, three important differences distinguish them. First, diseases affecting the descending pathways give rise to spasticity whereas diseases of motor neurons do not. Second, diseases affecting motor neurons directly result in denervation atrophy and reduced muscle volume, whereas this does not occur with damage to the descending pathway. Third, damage to descending systems tends to be distributed more diffusely in limb or face muscles and often affects large groups of muscles, for example the flexors. In contrast, degeneration in local groups of motor neurons tends to affect muscles in a patchy way and may even be limited to single muscles. Nerve lesions result in weakness that reflects the known distribution of individual nerves. We now consider the organization of the three levels of the motor hierarchy—the spinal cord, brain stem, and cerebral cortex—and how they control proximal and distal muscles.
- Figure 33-13 The motor nuclei of the spinal cord are arranged along a medial-lateral axis according to function. The medial nuclei contain the motor neurons innervating axial muscles of the neck and back; among the lateral nuclei the most medial motor neurons innervate proximal muscles while the most lateral innervate distal muscles. The medial motor nuclei are interconnected across several segments of the spinal cord by propriospinal neurons with long axons, whereas the lateral nuclei are interconnected across fewer segments by propriospinal neurons with shorter axons. Spinal Motor Neurons Execute Movement Primary afferent fibers from cutaneous and deep peripheral receptors (Chapter 22) branch profusely before terminating in the various laminae of the spinal gray matter, where they form connections with four types of neurons: (1) local interneurons, whose axons are confined to the same or adjacent spinal segments; (2) propriospinal neurons, whose axon terminals reach distant spinal segments; (3) projection neurons, whose axons ascend to higher brain centers; and (4) motor neurons, whose axons exit the nervous system to innervate muscles. We first consider the motor neurons and then the interneurons and propriospinal neurons that are important in motor control. The cell bodies of motor neurons that innervate individual muscles are clustered in motor neuron pools, or motor nuclei , which form longitudinal columns extending over one to four spinal segments. The spatial organization of the different motor nuclei follows a proximaldistal rule. According to this rule, motor nuclei innervating the most proximal muscles lie most medially within the spinal cord while those innervating more distal muscles are located progressively more laterally. Thus, for the arm, the motor nuclei innervating the axial, shoulder girdle, elbow, wrist, and digit muscles are arrayed from medial to lateral positions (Figure 33-13). The separation of motor neurons innervating axial and proximal muscles from those innervating distal muscles is maintained throughout the spinal cord. The functional specialization of medial and lateral motor nuclei is also reflected in the organization of the local interneurons of the spinal cord. Interneurons in the most medial parts of the intermediate zone of the spinal cord project to the medial motor nuclei that control axial muscles on both sides of the body. More laterally located interneurons project only to the motor neurons that innervate ipsilateral girdle muscles, while the most lateral ones synapse on motor neurons that innervate the most distal ipsilateral muscles (Figure 33-13). The axons of propriospinal neurons course up and down the white matter of the spinal cord and terminate on interneurons and motor neurons located several segments away from the cell bodies (Figure 33-13). Axons of medial propriospinal neurons run in the ventral and medial columns. They have long axons that branch extensively; some axons extend through the entire length of the spinal cord to coordinate movements of the neck and pelvis. This organization allows the axial muscles, which are innervated from many spinal segments, to be coordinated easily during postural adjustments. More laterally placed propriospinal neurons interconnect smaller numbers of segments and have less diffuse terminations. This explains the greater independence of action of more distal muscles, allowing a larger variety of muscle activation patterns. Although shoulder and elbow muscles are used to direct the hand in reaching for objects in different directions, shoulder and elbow motions are more stereotyped and less varied than those of the wrist and the elbow. Control of the digits requires the greatest degree of differentiation. Even the movements of a single digit require highly differentiated and coordinated contraction of many different muscles (Chapter 38).
- We have already noted that sensory information is integrated at all levels of the nervous system and causes appropriate motor responses that begin in the spinal cord with relatively simple muscle reflexes, extend into the brain stem with more complicated responses, and finally extend to the cerebrum, where the most complicated muscle skills are controlled. In this chapter, we discuss the control of muscle function by the spinal cord. Without the special neuronal circuits of the cord, even the most complex motor control systems in the brain could not cause any purposeful muscle movement. To give an example, there is no neuronal circuit anywhere in the brain that causes the specific to-and-fro movement of the legs that is required in walking. Instead, the circuits for these movements are in the cord, and the brain simply sends command signals to the spinal cord to set into motion the walking process. Let us not belittle the role of the brain, however, because the brain gives directions that control the sequential cord activities—to promote turning movements when they are required, to lean the body forward during acceleration, to change the movements from walking to jumping as needed, and to monitor continuously and control equilibrium. All this is done through “analytical” and “command” signals generated in the brain. But it also requires the many neuronal circuits of the spinal cord that are the objects of the commands. These circuits provide all but a small fraction of the direct control of the muscles. Spinal Animal and the Decerebrate Animal. Two types of experimental preparations have been especially useful in studying spinal cord function: (1) the spinal animal, in which the spinal cord is transected, frequently in the neck so that most of the cord still remains functional, and (2) the decerebrate animal, in which the brain stem is transected in the middle to lower part of the mesencephalon. Immediately after preparation of a spinal animal, most spinal cord function below the level of transection is severely depressed. After a few hours in rats and cats or a few days to weeks in monkeys, most of the intrinsic spinal cord functions return to nearly normal and provide a suitable experimental preparation for research study. In the decerebrate animal, the brain stem is transected at the middle to lower mesencephalic level, which blocks normal inhibitory signals from the higher control centers of the brain to the pontile and vestibular muscle control nuclei. This allows these nuclei to become tonically active, transmitting facilitatory signals to most of the spinal cord motor control circuits. The result is that the spinal cord motor reflexes become very excitable and, therefore, easy to activate by even the slightest sensory input signals to the cord. Using this preparation, one can study the intrinsic excitatory motor functions of the cord itself. Organization of the Spinal Cord for Motor Functions The cord gray matter is the integrative area for the cord reflexes. Figure 54–1 shows the typical organization of the cord gray matter in a single cord segment. Sensory signals enter the cord almost entirely through the sensory (posterior) roots. After entering the cord, every sensory signal travels to two separate destinations: (1) One branch of the sensory nerve terminates almost immediately in the gray matter of the cord and elicits local segmental cord reflexes and other local effects. (2) Another branch transmits signals to higher levels of the nervous system—to higher levels in the cord itself, to the brain stem, or even to the cerebral cortex, as described in earlier chapters. Each segment of the spinal cord (at the level of each spinal nerve) has several million neurons in its gray matter. Aside from the sensory relay neurons discussed in Chapters 47 and 48, the other neurons are of two types: (1) anterior motor neurons and (2) interneurons. Anterior Motor Neurons. Located in each segment of the anterior horns of the cord gray matter are several thousand neurons that are 50 to 100 per cent larger than most of the others and are called anterior motor neurons. They give rise to the nerve fibers that leave the cord by way of the anterior roots and directly innervate the skeletal muscle fibers. The neurons are of two types, alpha motor neurons and gamma motor neurons. Alpha Motor Neurons. The alpha motor neurons give rise to large type A alpha (Aa) motor nerve fibers, averaging 14 micrometers in diameter; these fibers branch many times after they enter the muscle and innervate the large skeletal muscle fibers. Stimulation of a single alpha nerve fiber excites anywhere from three to several hundred skeletal muscle fibers, which are collectively called the motor unit. Transmission of nerve impulses into skeletal muscles and their stimulation of the muscle motor units are discussed in Chapters 6 and 7. Gamma Motor Neurons. Along with the alpha motor neurons, which excite contraction of the skeletal muscle fibers, about one half as many much smaller gamma motor neurons are located in the spinal cord anterior horns. These gamma motor neurons transmit impulses through much smaller type A gamma (Ag) motor nerve fibers, averaging 5 micrometers in diameter, which go to small, special skeletal muscle fibers called intrafusal fibers, shown in Figure 54–2. These fibers constitute the middle of the muscle spindle, which helps control basic muscle “tone,” as discussed later in this chapter. Interneurons. Interneurons are present in all areas of the cord gray matter—in the dorsal horns, the anterior horns, and the intermediate areas between them, as shown in Figure 54–1. These cells are about 30 times as numerous as the anterior motor neurons. They are small and highly excitable, often exhibiting spontaneous activity and capable of firing as rapidly as 1500 times per second. They have many interconnections with one another, and many of them also synapse directly with the anterior motor neurons, as shown in Figure 54–1. The interconnections among the interneurons and anterior motor neurons are responsible for most of the integrative functions of the spinal cord that are discussed in the remainder of this chapter. Essentially all the different types of neuronal circuits described in Chapter 46 are found in the interneuron pool of cells of the spinal cord, including diverging, converging, repetitive-discharge, and other types of circuits. In this chapter, we examine many applications of these different circuits in the performance of specific reflex acts by the spinal cord. Only a few incoming sensory signals from the spinal nerves or signals from the brain terminate directly on the anterior motor neurons. Instead, almost all these signals are transmitted first through interneurons, where they are appropriately processed. Thus, in destinations: (1) One branch of the sensory nerve terminates almost immediately in the gray matter of the cord and elicits local segmental cord reflexes and other local effects. (2) Another branch transmits signals to higher levels of the nervous system—to higher levels in the cord itself, to the brain stem, or even to the cerebral cortex, as described in earlier chapters. Each segment of the spinal cord (at the level of each spinal nerve) has several million neurons in its gray matter. Aside from the sensory relay neurons discussed in Chapters 47 and 48, the other neurons are of two types: (1) anterior motor neurons and (2) interneurons. . Renshaw Cell Inhibitory System. Also located in the anterior horns of the spinal cord, in close association with the motor neurons, are a large number of small neurons called Renshaw cells. Almost immediately after the anterior motor neuron axon leaves the body of the neuron, collateral branches from the axon pass to adjacent Renshaw cells.These are inhibitory cells that transmit inhibitory signals to the surrounding motor neurons. Thus, stimulation of each motor neuron tends to inhibit adjacent motor neurons, an effect called lateral inhibition. This effect is important for the following major reason: The motor system uses this lateral inhibition to focus, or sharpen, its signals in the same way that the sensory system uses the same principle—that is, to allow unabated transmission of the primary signal in the desired direction while suppressing the tendency for signals to spread laterally. Multisegmental Connections from One Spinal Cord Level to Other Levels—Propriospinal Fibers More than half of all the nerve fibers that ascend and descend in the spinal cord are propriospinal fibers. These fibers run from one segment of the cord to another. In addition, as the sensory fibers enter the cord from the posterior cord roots, they bifurcate and branch both up and down the spinal cord; some of the branches transmit signals to only a segment or two, while others transmit signals to many segments. These ascending and descending propriospinal fibers of the cord provide pathways for the multisegmental reflexes described later in this chapter, including reflexes that coordinate simultaneous movements in the forelimbs and hind limbs.
- The Motor Unit and Muscle Action Gerald E. Loeb Claude Ghez … to move things is all that mankind can do, for such the sole executant is muscle, whether in whispering a syllable or in felling a forest. --Charles Sherrington, 1924 THE MAJOR CONSEQUENCE of the elaborate information processing that takes place in the brain is the contraction of skeletal muscles. Indeed, animals are distinguishable from plants by their ability to make precise, goal-directed movements of their body parts. The problem of deciding when and how to move is, to a large degree, the driving force behind the evolution of the nervous system. In this chapter we examine how the electrical and chemical signals used to convey information in the nervous system are ultimately converted into the forces and displacements that make up movement. In all but the most primitive animals movement is generated by specialized muscle cells. There are three general types of muscles: smooth muscle, used primarily for internal actions such as peristalsis and control of blood flow; cardiac muscle, used exclusively for pumping blood; and skeletal muscle, used primarily for moving bones. In this chapter we deal exclusively with the organization and neural control of mammalian skeletal muscles. Much of this chapter concerns the mechanical properties of muscles, tendons, and joints and the laws of physics that govern the motion of limbs. In order to perform a task the brain must solve a control problem that depends on these properties and laws. The difficulty of controlling a system with multiple linked segments can be appreciated by considering that, despite substantial computing power, industrial robots are relatively poor at compensating for unexpected perturbations that would pose no problem even for a simple animal. At least a part of the solution to the control problem resides in the muscles themselves. Our skeletal muscles are endowed with mechanical properties that contribute importantly to the grace, speed, efficiency, and robustness of our movements. Motor Neurons Convey Commands to Muscle Fibers Skeletal muscle is subdivided into parallel bundles of stringlike fascicles, which themselves are bundles of even smaller stringlike multinucleated cells called muscle fibers. A typical mammalian muscle fiber has a diameter of 50-100 μm and a length of 2-6 cm. Thus a typical muscle is composed of hundreds of thousands, even millions, of independent contractile elements arranged in parallel and, in longer muscles, in series. The main job of the motor nervous system is to control these elements in all of the muscles simultaneously so that the correct tension is applied to the skeleton to produce the desired movement. A typical muscle is controlled by about a hundred large motor neurons whose cell bodies lie in a distinct cluster called a motor nucleus in the spinal cord or brain stem (Figure 34-1). The axon of each motor neuron exits the spinal cord through a ventral root (or through a cranial nerve from the brain stem) and traverses progressively smaller branches of peripheral nerves until it enters the muscle it controls. There it branches widely to innervate anywhere from 100 to 1000 muscle fibers scattered over a substantial part of the muscle. Except during development, each muscle fiber is normally innervated by only one motor neuron in only one place, usually near its midpoint. The ensemble of muscle fibers innervated by a single motor neuron is called a muscle unit , and that ensemble together with its motor neuron is called a motor unit. The number of muscle fibers constituting a single motor unit varies greatly in muscles in different parts of the body (see Chapter 35). The functional connection between a motor neuron and a target muscle fiber is a chemical synapse called the end-plate (Chapter 11). End-plates are usually clustered into bands that extend across some or all of the muscle. The neuromuscular synapse formed by a motor neuron on a muscle fiber is large and filled with many vesicles containing the neurotransmitter acetylcholine. This synapse is constructed so that each action potential in the motor neuron releases sufficient transmitter to depolarize the postsynaptic membrane of the muscle fiber to its threshold for an action potential. The acetylcholine released from the presynaptic terminals is rapidly hydrolyzed by acetylcholinesterase, leaving the muscle fiber ready to respond again in an all-or-none manner to the next action potential. All of the muscle fibers innervated by the same motor neuron respond faithfully and synchronously to each action potential of the motor neuron. Once the postsynaptic membrane of the neuromuscular junction is depolarized to its threshold, an action potential propagates along the membrane of the muscle fiber (the sarcolemma). The action potential propagates relatively slowly (3-5 m/s) in both directions away from the end-plate region. A muscle fiber is electrically similar to a large-diameter, unmyelinated axon in that high transmembrane currents are required to propagate the action potential. These currents give rise to relatively large potential gradients in the extracellular fluid around the muscle fiber. Because a single action potential in a motor neuron can activate hundreds of muscle fibers in synchrony, the resulting currents sum to generate an electrical signal that is readily detectable outside the muscle itself. Furthermore, when more than minimal force is required, many motor neurons generate an asynchronous barrage of action potentials with overlapping action potentials arising in each muscle unit. The result is a complex pattern of electrical potentials (typically on the order of 100 μV in amplitude) that can be recorded as an electromyogram (EMG) using simple electrodes on the surface of the overlying skin. The relative timing and amplitude of these patterns recorded over particular muscles reflect closely the aggregate activity of motor neurons that innervate each muscle. Electromyographic signals are valuable for studying motor control and for diagnosing pathology in the motor systems and in the muscles themselves (see Chapter 35).
- Different views of the motor end plate. A, Longitudinal section through the end plate. B, Surface view of the end plate. C, Electron micrographic appearance of the contact point between a single axon terminal and the muscle fiber membrane.
- Acetylcholine channel. A, Closed state. B, After acetylcholine (Ach) has become attached and a conformational change has opened the channel, allowing sodium ions to enter the muscle fiber and excite contraction. Note the negative charges at the channel mouth that prevent passage of negative ions such as chloride ions.
- Excitation-contraction coupling in the muscle, showing (1) an action potential that causes release of calcium ions from the sarcoplasmic reticulum and then (2) reuptake of the calcium ions by a calcium pump.
- The Contractile Machinery of Muscle Fibers Is Organized Into Sarcomeres and Cross Bridges When viewed through the light microscope a single skeletal muscle fiber can be seen to contain many myofibrils , each of which has a longitudinally repeating pattern of dark and light bands called striations. The dark bands are constant in length, but the light bands tend to become longer or shorter as the muscle lengthens or shortens, respectively. Sarcomeres Are Composed of Interdigitated Thick and Thin Filaments Under the electron microscope individual myofibrils can be seen to consist of longitudinally repeated cylindrical units, called sarcomeres. Each sarcomere contains contractile proteins, organized into a regular interdigitated matrix of thick and thin filaments, and is bounded by Z disks (Figure 34-2). The changing banding pattern with muscle contraction evident in the light microscope results from the changing overlap between these filaments. The sarcomere is the functional unit of length in skeletal muscle. All myofibrils in all muscle fibers of a muscle tend to change length in concert as a result of the various noncontractile components that link them mechanically. The physiological range of length of each sarcomere is 1.5-3.5 μm. A muscle fiber with a 4-cm resting length would have about 20,000 sarcomeres in series. The thin filaments project in both directions from the Z disks, whereas the thick filaments are discontinuous and float in the middle of the sarcomere. The main constituent of each thin filament is a pair of polymerized actin monomers (F actin) arranged as a helix (Figure 34-2C). The thin filament also contains tropomyosin (a long filamentous protein that lies in the grooves formed by the paired strands of actin) and troponin (small molecular complexes that are attached to the tropomyosin filament at regular intervals). The thick filament is made up of about 250 myosin molecules entwined together along most of their lengths. The myosin molecules have globular heads on short stems that stick out from the sides of the thick filament in a staggered array, pointing away from a bare region in the middle of the filament where there are no heads (Figure 34-2C). Contractile Force Is Produced by Cross Bridges The thick and thin filaments comprise the contractile machinery of the muscle. In a contracting muscle adjacent thick and thin filaments slide past each other, propelled by cyclical interactions between the myosin heads of the thick filaments and binding sites on the actin of the adjacent thin filaments. This is the “sliding filament hypothesis” developed by A.F. Huxley and colleagues starting in the 1950s. Each globular myosin head contains an ATPase that converts the chemical energy of adenosine triphosphate (ATP) into mechanical energy, resulting in a “cocked” deformation of the myosin head (Figure 34-3). This stored mechanical energy can be released only after the myosin head attaches to a binding site on one of the adjacent thin filaments that has been activated by Ca2+ (a process described later). The attached head, or cross bridge , then acts like an oar, pulling the thin filament longitudinally in a direction that increases the overlap between the thick and thin filaments and shortening the muscle fiber. After a sliding motion of about 0.06 μm, the stress in the cross bridge is completely relieved and it can detach. Detachment is accompanied by recocking the head for reattachment to another binding site. The detachment of the myosin head from the actin molecule is an active process that uses energy derived from the hydrolysis of ATP into adenosine diphosphate (ADP) and phosphate in the presence of Ca2+. The process of attachment, rotation, and detachment therefore continues as long as Ca2+ and ATP are present in the cell in sufficient amounts. The stiff state of muscles after death known as rigor mortis results from cross bridges that cannot detach because ADP is not phosphorylated to replenish the ATP supply. Noncontractile Components in Muscle Fibers Provide Stability for the Contractile Elements Muscle fibers contain several structural elements whose mechanical properties ensure stable and efficient production and transmission of the active force generated by the contractile apparatus of the thin and thick filaments. In addition to the contractile myofilaments described above, a set of very thin and highly elastic filaments, the connecting filaments or connectins , extend from the ends of the thick filaments and attach on both flanking Z disks (see Figure 34-5 below). These connectins form a continuous elastic structure along the entire length of the muscle fiber, accounting for at least some of the springlike restoring force that can be measured when an inactive muscle is stretched passively (see below). The connecting filaments keep the thick and thin filaments aligned with respect to each other if the muscle is stretched past the overlap of the filaments. The remainder of the passive force is provided by endomysial connective tissue, a loose matrix of collagen that surrounds each muscle fiber and helps to distribute tension and sarcomere length changes evenly. Any active force generated by the contractile mechanism is independent of and additional to the passive force generated by these parallel elastic elements. At the ends of muscle fibers that insert onto connective tissue the last set of actin filaments attaches to specialized sites on the muscle fiber membrane where the tension is conveyed to invaginated strands of extracellular collagen in the connective tissue. Tendons and aponeuroses (see below) can stretch and store mechanical energy during muscle contraction, particularly if these in-series elastic elements are relatively long compared with the muscle fibers. Some muscles have long fascicles that are staggered bundles of shorter muscle fibers. The intrafascicular ends of these muscle fibers have a long tapered shape that provides a large surface area over which tensile force can be P.679 P.680 passed as shear force into the surrounding connective tissue. Some of the myopathies described in Chapter 35 may be related to failures of the noncontractile components of muscle.
- General Mechanism of Muscle Contraction The initiation and execution of muscle contraction occur in the following sequential steps. 1. An action potential travels along a motor nerve to its endings on muscle fibers. 2. At each ending, the nerve secretes a small amount of the neurotransmitter substance acetylcholine. 3. The acetylcholine acts on a local area of the muscle fiber membrane to open multiple “acetylcholinegated” channels through protein molecules floating in the membrane. 4. Opening of the acetylcholine-gated channels allows large quantities of sodium ions to diffuse to the interior of the muscle fiber membrane. This initiates an action potential at the membrane. 5. The action potential travels along the muscle fiber membrane in the same way that action potentials travel along nerve fiber membranes. 6. The action potential depolarizes the muscle membrane, and much of the action potential electricity flows through the center of the muscle fiber. Here it causes the sarcoplasmic reticulum to release large quantities of calcium ions that have been stored within this reticulum. 7. The calcium ions initiate attractive forces between the actin and myosin filaments, causing them to slide alongside each other, which is the contractile process. 8. After a fraction of a second, the calcium ions are pumped back into the sarcoplasmic reticulum by a Ca++ membrane pump, and they remain stored in the reticulum until a new muscle action potential comes along; this removal of calcium ions from the myofibrils causes the muscle contraction to cease. We now describe the molecular machinery of the muscle contractile process. Molecular Mechanism of Muscle Contraction Sliding Filament Mechanism of Muscle Contraction. Figure 6–4 demonstrates the basic mechanism of muscle contraction. It shows the relaxed state of a sarcomere (top) and the contracted state (bottom). In the relaxed state, the ends of the actin filaments extending from two successive Z discs barely begin to overlap one another. Conversely, in the contracted state, these actin filaments have been pulled inward among the myosin filaments, so that their ends overlap one another to their maximum extent. Also, the Z discs have been pulled by the actin filaments up to the ends of the myosin filaments. Thus, muscle contraction occurs by a sliding filament mechanism. But what causes the actin filaments to slide inward among the myosin filaments? This is caused by forces generated by interaction of the cross-bridges from the myosin filaments with the actin filaments. Under resting conditions, these forces are inactive, but when an action potential travels along the muscle fiber, this causes the sarcoplasmic reticulum to release large quantities of calcium ions that rapidly surround the myofibrils. The calcium ions in turn activate the forces between the myosin and actin filaments, and contraction begins. But energy is needed for the contractile process to proceed. This energy comes from highenergy bonds in the ATP molecule, which is degraded to adenosine diphosphate (ADP) to liberate the energy. In the next few sections, we describe what is known about the details of these molecular processes of contraction.
- Characteristics of Whole Muscle Contraction Many features of muscle contraction can be demonstrated by eliciting single muscle twitches. This can be accomplished by instantaneous electrical excitation of the nerve to a muscle or by passing a short electrical stimulus through the muscle itself, giving rise to a single, sudden contraction lasting for a fraction of a second. Isometric Versus Isotonic Contraction. Muscle contraction is said to be isometric when the muscle does not shorten during contraction and isotonic when it does shorten but the tension on the muscle remains constant throughout the contraction. Systems for recording the two types of muscle contraction are shown in Figure 6–11. In the isometric system, the muscle contracts against a force transducer without decreasing the muscle length, as shown on the right in Figure 6–11. In the isotonic system, the muscle shortens against a fixed load; this is illustrated on the left in the figure, showing a muscle lifting a pan of weights. The characteristics of isotonic contraction depend on the load against which the muscle contracts, as well as the inertia of the load. However, the isometric system records strictly changes in force of muscle contraction itself. Therefore, the isometric system is most often used when comparing the functional characteristics of different muscle types. Characteristics of Isometric Twitches Recorded from Different Muscles. The human body has many sizes of skeletal muscles—from the very small stapedius muscle in the middle ear, measuring only a few millimeters long and a millimeter or so in diameter, up to the very large quadriceps muscle, a half million times as large as the stapedius. Further, the fibers may be as small as 10 micrometers in diameter or as large as 80 micrometers. Finally, the energetics of muscle contraction vary considerably from one muscle to another. Therefore, it is no wonder that the mechanical characteristics of muscle contraction differ among muscles.
- Figure 16.5. Comparison of the force and fatigability of the three different types of motor units. In each case, the response reflects stimulation of a single motor neuron. (A) Change in muscle tension in response to a single motor neuron action potential. (B) Tension in response to repetitive stimulation of the motor neurons. (C) Response to repeated stimulation at a level that evokes maximum tension. The ordinate represents the force generated by each stimulus. Note the strikingly different fatigue rates. (After Burke et al., 1974.) Fast Versus Slow Muscle Fibers. As we discuss more fully in Chapter 84 on sports physiology, every muscle of the body is composed of a mixture of so-called fast and slow muscle fibers, with still other fibers gradated between these two extremes. The muscles that react rapidly are composed mainly of “fast” fibers with only small numbers of the slow variety. Conversely, the muscles that respond slowly but with prolonged contraction are composed mainly of “slow” fibers. The differences between these two types of fibers are as follows. Fast Fibers. (1) Large fibers for great strength of contraction.(2) Extensive sarcoplasmic reticulum for rapid release of calcium ions to initiate contraction. (3) Large amounts of glycolytic enzymes for rapid release of energy by the glycolytic process. (4) Less extensive blood supply because oxidative metabolism is of secondary importance. (5) Fewer mitochondria, also because oxidative metabolism is secondary. Slow Fibers. (1) Smaller fibers. (2) Also innervated by smaller nerve fibers. (3) More extensive blood vessel system and capillaries to supply extra amounts of oxygen. (4) Greatly increased numbers of mitochondria, also to support high levels of oxidative metabolism. (5) Fibers contain large amounts of myoglobin, an ironcontaining protein similar to hemoglobin in red blood cells. Myoglobin combines with oxygen and stores it until needed; this also greatly speeds oxygen transport to the mitochondria. The myoglobin gives the slow muscle a reddish appearance and the name red muscle, whereas a deficit of red myoglobin in fast muscle gives it the name white muscle
- Figure 16.6. The recruitment of motor neurons in the cat medial gastrocnemius muscle under different behavioral conditions. Slow (S) motor units provide the tension required for standing. Fast fatigue-resistant (FR) units provide the additional force needed for walking and running. Fast fatigable (FF) units are recruited for the most strenuous activities. (After Walmsley et al., 1978.) The Regulation of Muscle Force Increasing or decreasing the number of motor units active at any one time changes the amount of force produced by a muscle. In the 1960s, Elwood Henneman and his colleagues at Harvard Medical School found that steady increases in muscle tension could be produced by progressively increasing the activity of axons that provide input to the relevant pool of lower motor neurons. This gradual increase in tension results from the recruitment of motor units in a fixed order according to their size. By stimulating in an experimental animal either sensory nerves or upper motor pathways that project to a lower motorneuron pool while measuring the tension changes in the muscle, Henneman found that the smallest motor neurons in the pool are the only units activated by weak synaptic stimulation. When synaptic input increases, progressively larger motor neurons are recruited: As the synaptic activity driving a motor neuron pool increases, low threshold S units are recruited first, then FR units, and finally, at the highest levels of activity, the FF units. Since these original experiments, evidence for the orderly recruitment of motor units has been found in a variety of voluntary and reflexive movements. As a result, this systematic relationship has come to be known as the size principle . An illustration of how the size principle operates for the motor units of the medial gastrocnemius muscle in the cat is shown in Figure 16.6 . When the animal is standing quietly, the force measured directly from the muscle tendon is only a small fraction (about 5%) of the total force that the muscle can generate. The force is provided by the S motor units, which make up about 25% of the motor units in this muscle. When the cat begins to walk, larger forces are necessary: locomotor activities that range from slow walking to fast running require up to 25% of the muscle's total force capacity. This additional need is met by the recruitment of FR units. Only movements such as galloping and jumping, which are performed infrequently and for short periods, require the full power of the muscle; such demands are met by the recruitment of the FF units. Thus, the size principle provides a simple solution to the problem of grading muscle force: The combination of motor units activated by such orderly recruitment optimally matches the physiological properties of different motor unit types with the range of forces required to perform different motor tasks. The frequency of the action potentials generated by motor neurons also contributes to the regulation of muscle tension. The increase in force that occurs with increased firing rate reflects the summation of successive muscle contractions: The muscle fibers are activated by the next action potential before they have had time to completely relax, and the forces generated by the temporally overlapping contractions are summed ( Figure 16.7 ). The lowest firing rates during a voluntary movement are on the order of 8 per second ( Figure 16.8 ). As the firing rate of individual units rises to a maximum of about 20–25 per second in the muscle being studied here, the amount of force produced increases. At the highest firing rates, individual muscle fibers are in a state of “fused tetanus”—that is, the tension produced in individual motor units no longer has peaks and troughs that correspond to the individual twitches evoked by the motorneuron's action potentials. Under normal conditions, the maximum firing rate of motor neurons is less than that required for fused tetanus (see Figure 16.8 ). However, the asynchronous firing of different lower motor neurons provides a steady level of input to the muscle that causes the contraction of a relatively constant number of motor units and averages out the changes in tension due to contractions and relaxations of individual motor units. All this allows the resulting movements to be executed smoothly.
- Figure 36-3 A. The main components of the muscle spindle are intrafusal muscle fibers, afferent sensory fiber endings, and efferent motor fiber endings. The intrafusal fibers are specialized muscle fibers; their central regions are not contractile. The sensory fiber endings spiral around the central regions of the intrafusal fibers and are responsive to stretch of these fibers. Gamma motor neurons innervate the contractile polar regions of the intrafusal fibers. Contraction of the intrafusal fibers pulls on the central regions from both ends and changes the sensitivity of the sensory fiber endings to stretch. (Adapted from Hulliger 1984.) B. The muscle spindle contains three types of intrafusal fibers: dynamic nuclear bag, static nuclear bag, and nuclear chain fibers. A single Ia sensory fiber innervates all three types of fibers, forming a primary sensory ending. A group II sensory fiber innervates nuclear chain fibers and static bag fibers, forming a secondary sensory ending. Two types of motor neurons innervate different intrafusal fibers. Dynamic gamma motor neurons innervate only dynamic bag fibers; static gamma motor neurons innervate various combinations of chain and static bag fibers. (Adapted from Boyd 1980.) C. Selective stimulation of the two types of gamma motor neurons has different effects on the firing of the primary sensory endings in the spindle (the Ia fibers). Without gamma stimulation the Ia fiber shows a small dynamic response to muscle stretch and a modest increase in steady-state firing. When a static gamma motor neuron is stimulated the steady-state response of the Ia fiber increases but there is a decrease in the dynamic response. When a dynamic gamma motor neuron is stimulated the dynamic response of the Ia fiber is markedly enhanced but the steady-state response gradually returns to its original level. (Adapted from Brown and Matthews 1966.) Box 36-1 Muscle Spindles Muscle spindles are small encapsulated sensory receptors that have a spindle-like or fusiform shape and are located within the fleshy part of the muscle. Their main function is to signal changes in the length of the muscle within which they reside. Changes in the length of muscles are closely associated with changes in the angles of the joints that the muscles cross. Thus, muscle spindles can be used by the central nervous system to sense relative positions of the body segments. Each spindle has three main components: (1) a group of specialized intrafusal muscle fibers whose central regions are noncontractile; (2) large-diameter myelinated sensory endings that originate from the central regions of the intrafusal fibers; and (3) small-diameter myelinated motor endings that innervate the polar contractile regions of the intrafusal fibers (Figure 36-3A). When the intrafusal fibers are stretched, often referred to as “loading the spindle,” the sensory endings are also stretched and increase their firing rate. Because muscle spindles are arranged in parallel with the extrafusal muscle fibers that make up the main body of the muscle, the intrafusal fibers change in length as the whole muscle changes. Thus, when a muscle is stretched, the activity in the sensory endings of muscle spindles is increased. When a muscle shortens, the spindle is unloaded and the activity decreases. The motor innervation of the intrafusal muscle fibers comes from small-diameter motor neurons, called gamma motor neurons to distinguish them from the large-diameter alpha motor neurons that innervate the extrafusal muscle fibers. Contraction of the intrafusal muscle fibers does not contribute to the force of muscle contraction. Rather, activation of gamma motor neurons causes shortening of the polar regions of the intrafusal fibers. This in turn stretches the noncontractile central region from both ends, leading to an increase in firing rate of the sensory endings or to a greater likelihood that stretch of the muscle will cause the sensory ending to fire. Thus, the gamma motor neurons provide a mechanism for adjusting the sensitivity of the muscle spindles. The structure and functional behavior of muscle spindles is considerably more complicated than this simple description implies. When a muscle is stretched, there are two phases of the change in length: a dynamic phase, the period during which length is changing, and a static or steady-state phase, when the muscle has stabilized at a new length. Structural specializations within each component of the muscle spindles allow spindle afferents to signal aspects of each phase separately. There are two types of intrafusal muscle fibers: nuclear bag fibers and nuclear chain fibers. The bag fibers can be divided into two groups, dynamic and static. A typical spindle has 2 or 3 bag fibers and a variable number of chain fibers, usually about 5. Furthermore, there are two types of sensory fiber endings: a single primary ending and a variable number of secondary endings (up to 8). The primary (Ia fiber) ending spirals around the central region of all the intrafusal muscle fibers (Figure 36-3B). The secondary (group II fiber) endings are located adjacent to the central regions of the static bag and chain fibers. The gamma motor neurons can also be divided into two classes, dynamic and static. Dynamic gamma motor neurons innervate the dynamic bag fibers, while the static gamma motor neurons innervate the static bag and the chain fibers. This duality of structure is reflected in a duality of function. The steady-state or tonic discharge of both primary and secondary sensory endings signals the steady-state length of the muscle. The primary endings are, in addition, highly sensitive to the velocity of stretch, allowing them to provide information about the speed of movements. Because they are highly sensitive to small changes, primary endings provide quick information about unexpected changes in length, useful for generating quick corrective reactions. Increases in activity of dynamic gamma motor neurons increase the dynamic sensitivity of the primary endings but have no influence on the secondary endings. Increases in activity of static gamma motor neurons increase the tonic level of activity in both primary and secondary endings, decrease the dynamic sensitivity of primary endings, and can prevent the silencing of primary activity when a muscle is released from stretch (Figure 36-3C). Thus, the central nervous system can independently adjust the dynamic and static sensitivity of the sensory fibers from muscle spindles. Box 36-2 Selective Activation of Sensory Fibers from Muscle Sensory fibers are classified according to their diameter. Axons with larger diameters conduct action potentials more rapidly. Because each class of receptors gives rise to afferent fibers with diameters within a restricted range, this method of classification distinguishes to some extent the fibers that arise from the different groups of sensory receptors. The main groups of sensory fibers from muscle are listed in Table 36-1 (see Chapter 24 for the classification of sensory fibers from skin and joints). The organization of reflex pathways in the spinal cord has been established primarily by electrically stimulating the sensory fibers and recording evoked responses in different classes of neurons in the spinal cord. This method of activation has three advantages over natural stimulation. The timing of afferent input can be precisely established, the central responses evoked by different classes of sensory fibers can be assessed by grading the strength of the electrical stimulus, and certain classes of receptors can be activated in isolation (impossible in natural conditions). The strength of electrical stimuli required to activate a sensory fiber is measured relative to the strength required to activate the largest afferent fibers since the largest fibers have the lowest threshold for electrical activation. Thus group I fibers are usually activated over the range of one to two times the threshold of the largest afferents (with Ia fibers having, on average, a slightly lower threshold than Ib fibers). Most group II fibers are activated over the range of 2-5 times the threshold, while the small group III and IV fibers require stimulus strengths in the range of 10-50 times the threshold for activation. Reciprocal innervation of opposing muscles is not the only useful mode of coordination. Sometimes it is advantageous to contract the prime mover and the antagonist at the same time. Such co-contraction has the effect of stiffening the joint and is most useful when precision and joint stabilization are critical. An example of this phenomenon is the co-contraction of flexor and extensor muscles of the elbow immediately before catching a ball. The Ia inhibitory interneurons receive both excitatory and inhibitory signals from all of the major descending pathways (Figure 36-5A). By changing the balance of excitatory and inhibitory inputs onto these interneurons, supraspinal centers can reduce reciprocal inhibition and enable co-contraction, thus controlling the relative amount of joint stiffness to meet the requirements of the motor act. The activity of spinal motor neurons is also regulated by another important class of inhibitory interneurons, the Renshaw cells (Figure 36-5B). Renshaw cells are excited by collaterals of the axons of motor neurons, and they make inhibitory synaptic connections to several populations of motor neurons, including the same motor neurons that excite them, and to the Ia inhibitory interneurons. The connections of Renshaw cells to motor neurons form a negative feedback system that may help stabilize the firing rate of the motor neurons, while the connections to the Ia inhibitory interneurons may regulate the strength of reciprocal inhibition to antagonistic motor neurons. In addition, Renshaw cells receive significant synaptic input from descending pathways and distribute inhibition to task-related groups of motor neurons and Ia interneurons. Thus, it is likely that they contribute to establishing the pattern of transmission in divergent group Ia pathways according to the motor task.
- Figure 16.9. Stretch reflex circuitry. (A) Diagram of muscle spindle, the sensory receptor that initiates the stretch reflex. (B) Stretching a muscle spindle leads to increased activity in Ia afferents and an increase in the activity of α motor neurons that innervate the same muscle. Ia afferents also excite the motor neurons that innervate synergistic muscles, and inhibit the motor neurons that innervate antagonists (see also Figure 1.5 ). (C) The stretch reflex operates as a negative feedback loop to regulate muscle length. The Spinal Cord Circuitry Underlying Muscle Stretch Reflexes The local circuitry within the spinal cord mediates a number of sensory motor reflex actions. The simplest of these reflex arcs entails the response to musclestretch, which provides direct excitatory feedback to the motor neurons innervating the muscle that has been stretched ( Figure 16.9 ). As already mentioned, the sensory signal for the stretch reflex originates in muscle spindles , sensory receptors embedded within most muscles (see previous section and Chapter 9 ). The spindles comprise 8–10 intrafusal fibers arranged in parallel with the extrafusal fibers that make up the bulk of the muscle ( Figure 16.9A ). Large-diameter sensory fibers, called Ia afferents, are coiled around the central part of the spindle. These afferents are the largest axons in peripheral nerves and, since action potential conduction velocity is a direct function of axon diameter (see Chapters 2 and 3 ), they allow for very rapid adjustments in this reflex arc when the muscle is stretched. The stretch imposed on the muscle deforms the intrafusal muscle fibers, which in turn initiate action potentials by activating mechanically gated ion channels in the afferent axons coiled around the spindle. The centrally projecting branch of the sensory neuron forms monosynaptic excitatory connections with the α motor neurons in the ventral horn of the spinal cord that innervate the same (homonymous) muscle and, via local circuit neurons, inhibitory connections with the α motor neurons of antagonistic (heteronymous) muscles. This arrangement is an example of what is called reciprocal innervation and results in rapid contraction of the stretched muscle and simultaneous relaxation of the antagonist muscle. All of this leads to especially rapid and efficient responses to changes in the length or tension in the muscle ( Figure 16.9B ). The excitatory pathway from a spindle to the α motor neurons innervating the same muscle is unusual in that it is a monosynaptic reflex; in most cases, sensory neurons from the periphery do not contact the lower motor neuron directly but exert their effects through local circuit neurons. This monosynaptic reflex arc is variously referred to as the “stretch,” “deep tendon,” or “myotatic reflex,” and it is the basis of the knee, ankle, jaw, biceps, or triceps responses tested in a routine neurological examination. The tap of the reflex hammer on the tendon stretches the muscle and therefore excites a volley of activity from the muscle spindles in the afferent axons. The afferent volley is relayed to the α motor neurons in the brainstem or spinal cord, and an efferent volley returns to the muscle (see Figure 1.5 ). Since muscles are always under some degree of stretch, this reflex circuit is normally responsible for the steady level of tension in muscles called muscle tone . Changes in muscle tone occur in a variety of pathological conditions, and it is these changes that are assessed by examination of tendon reflexes. In terms of engineering principles, the stretch reflex arc is a negative feedback loop used to maintain muscle length at a desired value ( Figure 16.9C ). The appropriate muscle length is specified by the activity of descending pathways that influence the motor neuron pool. Deviations from the desired length are detected by the muscle spindles, since increases or decreases in the stretch of the intrafusal fibers alter the level of activity in the sensory fibers that innervate the spindles. These changes lead in turn to adjustments in the activity of the α motor neurons, returning the muscle to the desired length by contracting the stretched muscle and relaxing the opposed muscle group, and by restoring the level of spindle activity to what it was before. The smaller γ motor neurons control the functional characteristics of the muscle spindles by modulating their level of excitability. As already described, when the muscle is stretched, the spindle is also stretched and the rate of discharge in the afferent fibers increased. When the muscle shortens, however, the spindle is relieved of tension, or “unloaded,” and the sensory axons that innervate the spindle might therefore be expected to fall silent during contraction. However, they remain active. The γ motor neurons terminate on the contractile poles of the intrafusal fibers, and the activation of these neurons causes intrafusal fiber contraction—in this way maintaining the tension on the middle (or equatorial region) of the intrafusal fibers where the sensory axons terminate. Thus, co-activation of the α and γ motor neurons allows spindles to function (i.e., send information centrally) at all muscle lengths during movements and postural adjustments
- Figure 16.11. Comparison of the function of muscle spindles and Golgi tendon organs. (A) Golgi tendon organs are arranged in series with extrafusal muscle fibers because of their location at the junction of muscle and tendon. (B) The two types of muscle receptors, the muscle spindles (1) and the Golgi tendon organs (2), have different responses to passive muscle stretch ( top ) and active muscle contraction ( bottom ). Both afferents discharge in response to passively stretching the muscle, although the Golgi tendon organ discharge is much less than that of the spindle. When the extrafusal muscle fibers are made to contract by stimulation of their motor neurons, however, the spindle is unloaded and therefore falls silent, whereas the rate of Golgi tendon organ firing increases. (B after Patton, 1965 .) Other Afferent Feedback that Affects Motor Performance Another sensory receptor that is important in the reflex regulation of motor unit activity is the Golgi tendon organ . Golgi tendon organs are encapsulated afferent nerve endings located at the junction of the muscle and tendon ( Figure 16.11A ; see also Table 9.1 ). Each tendon organ is related to a single group Ib sensory axon (the Ib axons being slightly smaller than the Ia axons that innervate the muscle spindles). In contrast to the parallel arrangement of extrafusal muscle fibers and spindles, Golgi tendon organs are in series with the extrafusal muscle fibers. When a muscle is passively stretched, most of the change in length occurs in the muscle fibers, since they are more elastic than the fibrils of the tendon. When a muscle actively contracts, however, the force acts directly on the tendon, leading to an increase in the tension of the collagen fibrils in the tendon organ and compression of the intertwined sensory receptors. As a result, Golgi tendon organs are exquisitely sensitive to increases in muscle tension that arise from muscle contraction but, unlike spindles, are relatively insensitive to passive stretch ( Figure 16.11B ). The Ib axons from Golgi tendon organs contact inhibitory local circuit neurons in the spinal cord (called Ib inhibitory interneurons) that synapse, in turn, with the α motor neurons that innervate the same muscle. The Golgi tendon circuit is thus a negative feedback system that regulates muscle tension; it decreases the activation of a muscle when exceptionally large forces are generated and this way protects the muscle's integrity. This reflex circuit also operates at reduced levels of muscle force, counteracting small changes in muscle tension by increasing or decreasing the inhibition of α motor neurons. Under these conditions, the Golgi tendon system tends to maintain a steady level of force, counteracting effects that diminish muscle force (such as fatigue). In short, if the muscle spindle system is considered a feedback system that monitors and maintains muscle length , then the Golgi tendon system is a feedback system that monitors and maintains muscle force . Like the muscle spindle system, the Golgi tendon organ system is not a closed loop. The Ib inhibitory interneurons also receive synaptic inputs from a variety of other sources, including cutaneous receptors, joint receptors, muscle spindles, and descending upper motor neuron pathways ( Figure 16.12 ). Acting in concert, these inputs regulate the responsiveness of Ib interneurons to activity arising in Golgi tendon organs
- Figure 36-12 Alpha and gamma motor neurons are coactivated during voluntary movements. A. Coactivation of alpha and gamma motor neurons by a motor command allows feedback from muscle spindles to reinforce the activation of the alpha motor neurons. Since any disturbance during the movement alters the length of the muscle and changes the activity in the muscle spindles, altering the spindle input to the alpha motor neuron compensates for the disturbance. B. Recordings from the primary sensory fiber of a spindle during slow flexion of a finger show the spindle's discharge increasing. This increase in discharge rate depends on alpha-gamma coactivation. If the gamma motor neurons were not active the spindle would slacken and its discharge rate would decrease as the muscle shortened. (Adapted from Vallbo 1981.) Stretch Reflexes Reinforce Central Commands for Movements Proprioceptive reflexes can modulate motor output during voluntary movements because they function not only as discrete reflexes but also as closed feedback loops (Figure 36-12A). For example, stretch of a muscle produces an increase in spindle discharge, leading to muscle contraction and a consequent shortening of the muscle. But this muscle shortening leads to a decrease in spindle discharge, a reduction of muscle contraction, and a lengthening of the muscle. Thus, the stretch reflex loop acts continuously, tending to keep muscle length close to a desired or reference value. This is referred to as feedback because the output of the system (a change in muscle length) is “fed back” and becomes the input. The stretch reflex is a negative feedback system because it tends to counteract or reduce deviations from the reference value of the regulated variable. In 1963 Ragnar Granit proposed that, in voluntary movements, the reference value is set by descending signals that act on both the alpha and gamma motor neurons. The rate of firing of alpha motor neurons is set to produce the desired shortening of the muscle, while the rate of firing of gamma motor neurons is set to produce an equivalent shortening of the intrafusal fibers of the muscle spindle. If shortening of the whole muscle is less than what is required by a task, as when a load is greater than predicted, spindle afferent fibers increase their firing rate since the contracting intrafusal fibers are stretched (loaded) by the relatively longer length of the whole muscle. If shortening is more than is necessary, spindle afferents decrease their firing rate since the intrafusal fibers are relatively slackened (unloaded). Thus, one function of the monosynaptic excitatory pathway may be to provide a compensatory mechanism for unexpected alterations in load encountered by the muscles. Although direct evidence for this role of proprioceptive reflexes is lacking, there is strong evidence that alpha and gamma motor neurons are coactivated during voluntary movements by human subjects. In the late 1960s Åke Vallbo and Karl-Erik Hagbarth developed a technique known as microneurography to record from the largest afferents in peripheral nerves. Vallbo later showed that during slow movements of the fingers the primary spindle afferents (group Ia fibers) from the contracting muscles increase their rate of firing even when the muscle shortens as it contracts (Figure 36-12B). The only explanation for this finding is that the gamma motor neurons are active in synchrony with alpha motor neurons. Further, when subjects attempted to make slow movements at a constant velocity, the trajectory of the movements showed small deviations from a constant velocity—at times the muscle shortened quickly and at others times more slowly. The firing of the Ia sensory fiber mirrored the irregularities in the trajectory. When the velocity of flexion increased transiently, the rate of firing in the Ia fibers decreased because the muscle was shortening more rapidly and therefore exerted less tension on the intrafusal fibers. When the velocity decreased, Ia fiber firing increased because the muscle was shortening more slowly and therefore relative tension on the intrafusal fibers increased. This information can be used by the nervous system to compensate for irregularities in the movement trajectory by exciting the alpha motor neurons. Thus the stretch reflex may function as a servomechanism , that is, a feedback loop in which the output variable (actual muscle length) automatically follows a changing reference value (intended muscle length). In theory this mechanism could permit the nervous system to produce a movement of a given distance without having to know in advance the actual load or weight being moved. In practice, however, the stretch reflex pathways do not exert sufficient influence over motor neurons to overcome large unexpected loads. This is immediately obvious if we consider what happens when we attempt to lift a heavy suitcase that we thought was empty. We have to pause briefly and make a new movement with much greater muscle activation. Stretch reflex pathways therefore provide a mechanism for compensating for small changes in load and intrinsic irregularities in the muscle contraction. This action is mediated by both monosynaptic and long-loop pathways, with the relative contribution of each pathway dependent on the muscle and the task.
- Reflexes of Posture and Locomotion Postural and Locomotive Reflexes of the Cord Positive Supportive Reaction. Pressure on the footpad of a decerebrate animal causes the limb to extend against the pressure applied to the foot. Indeed, this reflex is so strong that if an animal whose spinal cord has been transected for several months—that is, after the reflexes have become exaggerated—is placed on its feet, the reflex often stiffens the limbs sufficiently to support the weight of the body. This reflex is called the positive supportive reaction. The positive supportive reaction involves a complex circuit in the interneurons similar to the circuits responsible for the flexor and cross extensor reflexes. The locus of the pressure on the pad of the foot determines the direction in which the limb will extend; pressure on one side causes extension in that direction, an effect called the magnet reaction. This helps keep an animal from falling to that side. Cord “Righting” Reflexes. When a spinal animal is laid on its side, it will make incoordinate movements trying to raise itself to the standing position. This is called the cord righting reflex. Such a reflex demonstrates that some relatively complex reflexes associated with posture are integrated in the spinal cord. Indeed, an animal with a well-healed transected thoracic cord between the levels for forelimb and hindlimb innervation can right itself from the lying position and even walk using its hindlimbs in addition to its forelimbs. In the case of an opossum with a similar transection of the thoracic cord, the walking movements of the hindlimbs are hardly different from those in a normal opossum—except that the hindlimb walking movements are not synchronized with those of the forelimbs. Stepping and Walking Movements Rhythmical Stepping Movements of a Single Limb. Rhythmical stepping movements are frequently observed in the limbs of spinal animals. Indeed, even when the lumbar portion of the spinal cord is separated from the remainder of the cord and a longitudinal section is made down the center of the cord to block neuronal connections between the two sides of the cord and between the two limbs, each hindlimb can still perform individual stepping functions. Forward flexion of the limb is followed a second or so later by backward extension. Then flexion occurs again, and the cycle is repeated over and over. This oscillation back and forth between flexor and extensor muscles can occur even after the sensory nerves have been cut, and it seems to result mainly from mutually reciprocal inhibition circuits within the matrix of the cord itself, oscillating between the neurons controlling agonist and antagonist muscles. The sensory signals from the footpads and from the position sensors around the joints play a strong role in controlling foot pressure and frequency of stepping when the foot is allowed to walk along a surface. In fact, the cord mechanism for control of stepping can be even more complex. For instance, if the top of the foot encounters an obstruction during forward thrust, the forward thrust will stop temporarily; then, in rapid sequence, the foot will be lifted higher and proceed forward to be placed over the obstruction. This is the stumble reflex. Thus, the cord is an intelligent walking controller. Reciprocal Stepping of Opposite Limbs. If the lumbar spinal cord is not split down its center, every time stepping occurs in the forward direction in one limb, the opposite limb ordinarily moves backward.This effect results from reciprocal innervation between the two limbs. Diagonal Stepping of All Four Limbs—“Mark Time” Reflex. If a well-healed spinal animal (with spinal transection in the neck above the forelimb area of the cord) is held up from the floor and its legs are allowed to dangle, as shown in Figure 54–12, the stretch on the limbs occasionally elicits stepping reflexes that involve all four limbs. In general, stepping occurs diagonally between the forelimbs and hindlimbs.This diagonal response is another manifestation of reciprocal innervation, this time occurring the entire distance up and down the cord between the forelimbs and hindlimbs. Such a walking pattern is called a mark time reflex. Galloping Reflex. Another type of reflex that occasionally develops in a spinal animal is the galloping reflex, in which both forelimbs move backward in unison while both hindlimbs move forward.This often occurs when almost equal stretch or pressure stimuli are applied to the limbs on both sides of the body at the same time; unequal stimulation elicits the diagonal walking reflex. This is in keeping with the normal patterns of walking and galloping, because in walking, only one forelimb and one hindlimb at a time are stimulated, which would predispose the animal to continue walking. Conversely, when the animal strikes the ground during galloping, both forelimbs and both hindlimbs are stimulated about equally; this predisposes the animal to keep galloping and, therefore, continues this pattern of motion. Scratch Reflex An especially important cord reflex in some animals is the scratch reflex, which is initiated by itch or tickle sensation. It involves two functions: (1) a position sense that allows the paw to find the exact point of irritation on the surface of the body, and (2) a to-and-fro scratching movement. The position sense of the scratch reflex is a highly developed function. If a flea is crawling as far forward as the shoulder of a spinal animal, the hind paw can still find its position, even though 19 muscles in the limb must be contracted simultaneously in a precise pattern to bring the paw to the position of the crawling flea. To make the reflex even more complicated, when the flea crosses the midline, the first paw stops scratching and the opposite paw begins the to-and-fro motion and eventually finds the flea. The to-and-fro movement, like the stepping movements of locomotion, involves reciprocal innervation circuits that cause oscillation. Spinal Cord Reflexes That Cause Muscle Spasm In human beings, local muscle spasm is often observed. In many, if not most, instances, localized pain is the cause of the local spasm. Muscle Spasm Resulting from a Broken Bone. One type of clinically important spasm occurs in muscles that surround a broken bone. This results from pain impulses initiated from the broken edges of the bone, which cause the muscles that surround the area to contract tonically. Pain relief obtained by injecting a local anesthetic at the broken edges of the bone relieves the spasm; a deep general anesthetic of the entire body, such as ether anesthesia, also relieves the spasm. One of these two anesthetic procedures is often necessary before the spasm can be overcome sufficiently for the two ends of the bone to be set back into their appropriate positions. Abdominal Muscle Spasm in Peritonitis. Another type of local spasm caused by cord reflexes is abdominal spasm resulting from irritation of the parietal peritoneum by peritonitis. Here again, relief of the pain caused by the peritonitis allows the spastic muscle to relax. The same type of spasm often occurs during surgical operations; for instance, during abdominal operations, pain impulses from the parietal peritoneum often cause the abdominal muscles to contract extensively, sometimes extruding the intestines through the surgical wound. For this reason, deep anesthesia is usually required for intraabdominal operations. Muscle Cramps. Still another type of local spasm is the typical muscle cramp. Electromyographic studies indicate that the cause of at least some muscle cramps is as follows: Any local irritating factor or metabolic abnormality of a muscle, such as severe cold, lack of blood flow, or overexercise, can elicit pain or other sensory signals transmitted from the muscle to the spinal cord, which in turn cause reflex feedback muscle contraction. The contraction is believed to stimulate the same sensory receptors even more, which causes the spinal cord to increase the intensity of contraction. Thus, positive feedback develops, so that a small amount of initial irritation causes more and more contraction until a fullblown muscle cramp ensues. Autonomic Reflexes in the Spinal Cord Many types of segmental autonomic reflexes are integrated in the spinal cord, most of which are discussed in other chapters. Briefly, these include (1) changes in vascular tone resulting from changes in local skin heat (see Chapter 73); (2) sweating, which results from localized heat on the surface of the body (see Chapter 73); (3) intestinointestinal reflexes that control some motor functions of the gut (see Chapter 62); (4) peritoneointestinal reflexes that inhibit gastrointestinal motility in response to peritoneal irritation (see Chapter 66); and (5) evacuation reflexes for emptying the full bladder (see Chapter 31) or the colon (see Chapter 63). In addition, all the segmental reflexes can at times be elicited simultaneously in the form of the so-called mass reflex, described next. Figure 54–12 Diagonal stepping movements exhibited by a spinal animal. 684 Unit XI The Nervous System: C. Motor and Integrative Neurophysiology Mass Reflex. In a spinal animal or human being, sometimes the spinal cord suddenly becomes excessively active, causing massive discharge in large portions of the cord.The usual stimulus that causes this is a strong pain stimulus to the skin or excessive filling of a viscus, such as overdistention of the bladder or the gut. Regardless of the type of stimulus, the resulting reflex, called the mass reflex, involves large portions or even all of the cord. The effects are (1) a major portion of the body’s skeletal muscles goes into strong flexor spasm; (2) the colon and bladder are likely to evacuate; (3) the arterial pressure often rises to maximal values, sometimes to a systolic pressure well over 200 mm Hg; and (4) large areas of the body break out into profuse sweating. Because the mass reflex can last for minutes, it presumably results from activation of great numbers of reverberating circuits that excite large areas of the cord at once. This is similar to the mechanism of epileptic seizures, which involve reverberating circuits that occur in the brain instead of in the cord. Spinal Cord Transection and Spinal Shock When the spinal cord is suddenly transected in the upper neck, at first, essentially all cord functions, including the cord reflexes, immediately become depressed to the point of total silence, a reaction called spinal shock. The reason for this is that normal activity of the cord neurons depends to a great extent on continual tonic excitation by the discharge of nerve fibers entering the cord from higher centers, particularly discharge transmitted through the reticulospinal tracts, vestibulospinal tracts, and corticospinal tracts. After a few hours to a few weeks, the spinal neurons gradually regain their excitability. This seems to be a natural characteristic of neurons everywhere in the nervous system—that is, after they lose their source of facilitatory impulses, they increase their own natural degree of excitability to make up at least partially for the loss. In most nonprimates, excitability of the cord centers returns essentially to normal within a few hours to a day or so, but in human beings, the return is often delayed for several weeks and occasionally is never complete; conversely, sometimes recovery is excessive, with resultant hyperexcitability of some or all cord functions. Some of the spinal functions specifically affected during or after spinal shock are the following: 1. At onset of spinal shock, the arterial blood pressure falls instantly and drastically—sometimes to as low as 40 mm Hg—thus demonstrating that sympathetic nervous system activity becomes blocked almost to extinction. The pressure ordinarily returns to normal within a few days, even in human beings. 2. All skeletal muscle reflexes integrated in the spinal cord are blocked during the initial stages of shock. In lower animals, a few hours to a few days are required for these reflexes to return to normal; in human beings, 2 weeks to several months are sometimes required. In both animals and humans, some reflexes may eventually become hyperexcitable, particularly if a few facilitatory pathways remain intact between the brain and the cord while the remainder of the spinal cord is transected. The first reflexes to return are the stretch reflexes, followed in order by the progressively more complex reflexes: flexor reflexes, postural antigravity reflexes, and remnants of stepping reflexes. 3. The sacral reflexes for control of bladder and colon evacuation are suppressed in human beings for the first few weeks after cord transection, but in most cases they eventually return. These effects are discussed in Chapters 31 and 66.
- Figure 37-3 Rhythmic activity for walking is generated by networks of neurons in the spinal cord. The existence of such spinal networks was first demonstrated by Thomas Graham Brown in 1911. Graham Brown developed an experimental animal system in which dorsal root nerves were cut so that sensory information from the limbs could not reach the spinal cord. An original record from Graham Brown's study shows that rhythmic alternating contractions of ankle flexor (tibialis anterior) and extensor (gastrocnemius) muscles begin very soon after transection of the spinal cord. The Motor Pattern for Stepping in Mammals Is Produced at the Spinal Level Transection of the spinal cord of quadrupeds initially causes complete paralysis of the hind legs. However, this operation does not permanently abolish the capacity of hind legs to make stepping movements; hind leg stepping often recovers spontaneously over a period of a few weeks, particularly if the transection is made in young animals. Recovery of stepping in adult cats can be facilitated by daily training on a treadmill combined with nonspecific cutaneous stimulation of the perineal region. In chronic spinal cats electromyographic records from hind leg muscles during stepping are very similar to those from normal walking animals. Many of the reflex responses occurring in normal animals can be evoked in spinal animals. Neuronal Networks Within the Spinal Cord Generate Rhythmic Alternating Activity in Flexor and Extensor Muscles From the studies by Graham Brown early in the twentieth century we know that the isolated spinal cord can generate rhythmic bursts of reciprocal activity in flexor and extensor motor neurons of the hind legs even in the absence of sensory input (Figure 37-3). Graham Brown proposed that contractions in the flexor and extensor muscles were controlled by two systems of neurons, which he termed half-centers , that mutually inhibit each other. He suggested that the switching of activity from one half-center to the other depended on fatigue in the inhibitory connections. Neuronal Networks Within the Spinal Cord Generate Rhythmic Alternating Activity in Flexor and Extensor Muscles From the studies by Graham Brown early in the twentieth century we know that the isolated spinal cord can generate rhythmic bursts of reciprocal activity in flexor and extensor motor neurons of the hind legs even in the absence of sensory input (Figure 37-3). Graham Brown proposed that contractions in the flexor and extensor muscles were controlled by two systems of neurons, which he termed half-centers , that mutually inhibit each other. He suggested that the switching of activity from one half-center to the other depended on fatigue in the inhibitory connections.
- Figure 16.14. The cycle of locomotion for terrestrial mammals (a cat in this instance) is organized by central pattern generators. (A) The step cycle, showing leg flexion (F) and extension (E) and their relation to the swing and stance phases of locomotion. EMG indicates electromyographic recordings. (B) Comparison of the stepping movements for different gaits. Brown bars, foot lifted (swing phase); gray bars, foot planted (stance phase). (C) Transection of the spinal cord at the thoracic level isolates the hindlimb segments of the cord. The hindlimbs are still able to walk on a treadmill after recovery from surgery, and reciprocal bursts of electrical activity can be recorded from flexors during the swing phase and from extensors during the stance phase of walking. (AfterPearson, 1976.) Spinal Cord Circuitry and Locomotion The contribution of local circuitry to motor control is not, of course, limited to reflexive responses to sensory inputs. Studies of rhythmic movements such aslocomotion and swimming in animal models ( Box A ) have demonstrated that local circuits in the spinal cord are fully capable of controlling the timing and coordination of such complex patterns of movement, and of adjusting them in response to altered circumstances ( Box B ). A good example is locomotion (walking, running, etc.). The movement of a single limb during locomotion can be thought of as a cycle consisting of two phases: a stance phase, during which the limb is extended and placed in contact with the ground to propel humans or other bipeds forward; and a swing phase, during which the limb is flexed to leave the ground and then brought forward to begin the next stance phase ( Figure 16.14A ). Increases in the speed of locomotion reduce the amount of time it takes to complete a cycle, and most of the change in cycle time is due to a shortening of the stance phase; the swing phase remains relatively constant over a wide range of locomotor speeds. In quadrupeds, changes in locomotor speed are also accompanied by changes in the sequence of limb movements. At low speeds, for example, there is a back-to-front progression of leg movements, first on one side and then on the other. As the speed increases to a trot, the movements of the right forelimb and left hindlimb are synchronized (as are the movements of the left forelimb and right hindlimb). At the highest speeds (gallop), the movements of the two front legs are synchronized, as are the movements of the two hindlimbs ( Figure 16.14B ). Given the precise timing of the movement of individual limbs and the coordination between limbs that is required in this process, it is natural to assume thatlocomotion is accomplished by higher centers that organize the spatial and temporal activity patterns of the individual limbs. However, following transection of the spinal cord at the thoracic level, a cat's hindlimbs will still make coordinated locomotor movements if the animal is supported and placed on a moving treadmill ( Figure 16.14C ). Under these conditions, the speed of locomotor movements is determined by the speed of the treadmill, suggesting that the movement is nothing more than a reflexive response to stretching the limb muscles. This possibility is ruled out, however, by experiments in which the dorsal roots are also sectioned. Although the speed of walking is slowed and the movements are less coordinated than under normal conditions, appropriate locomotor movements are still observed. These and other observations in experimental animals show that the basic rhythmic patterns of limb movement during locomotion are not dependent on sensory input; nor are they dependent on input from descending projections from higher centers. Rather, each limb appears to have its own central pattern generator —an oscillatory spinal cord local circuit responsible for the alternating flexion and extension of the limb during locomotion. Under normal conditions, the central pattern generators for the limbs are variably coupled to each other by additional local circuits in order to achieve the different sequences of movements that occur at different speeds. Although some locomotor movements can also be elicited in humans following damage to descending pathways, these are considerably less effective than the movements seen in the cat. The reduced ability of the transected spinal cord to mediate rhythmic stepping movements in humans presumably reflects an increased dependence of local circuitry on upper motor neuron pathways. Perhaps bipedal locomotion carries with it requirements for postural control greater than can be accommodated by spinal cord circuitry alone. Whatever the explanation, the basic oscillatory circuits that control such rhythmic behaviors as flying, walking, and swimming in many animals also play an important part in human locomotion
- B. Profiles of electrical activity in some of the hind leg flexor and extensor muscles in the cat during stepping. Although activity of flexor and extensor muscles generally occurs during swing and stance, respectively, the overall pattern of activity is complex in both timing and amplitude. The positions of the muscles are illustrated on the right. IP = iliopsoas; LG and MG = lateral and medial gastrocnemius; PB = posterior biceps; RF = rectus femorus; Sartm and Sarta = medial and anterior sartorius; SOL = soleus; ST = semitendinosus; TA = tibialis anterior; VL, VM, and VI = vastus lateralis, medialis, and intermedialis.
- Figure 37-4 Elements of the central pattern generator are revealed by electrical stimulation of high-threshold cutaneous and muscle afferents (flexor reflex afferents, FRA) in spinal cats treated with L-DOPA (L-dihydrox-yphenylalanine) and nialamide. A. Brief stimulation of ipsilateral FRA evokes a short sequence of rhythmic activity in flexor and extensor motor neurons. (Adapted from Jankowska et al. 1967a.) B. Reciprocal inhibition between interneurons in the pathways mediating long-latency reflexes from the ipsilateral and contralateral FRA. This half-center organization of the flexor and extensor interneurons is likely the basis for central rhythm generation for stepping. MN = motor neuron. The half-center hypothesis was supported by studies in the 1960s of the effects of the drug L-dihydroxyphenylalanine (L-DOPA, a precursor for the monoamine transmitters dopamine and norepinephrine) in spinal cats. After the cats were treated with L-DOPA, brief trains of electrical stimuli were applied to small-diameter cutaneous and muscle afferents. These evoked long-lasting bursts of activity in either flexor or extensor motor neurons depending on whether ipsilateral or contralateral nerves were stimulated. Collectively the group of small-diameter afferents producing these effects are referred to as flexor reflex afferents (FRA). Additional administration of nialamide (a drug prolonging the action of released norepinephrine in the spinal cord) often resulted in short sequences of rhythmic reciprocal activity in flexor and extensor motor neurons (Figure 37-4A). The system of interneurons generating the flexor bursts was found to inhibit the system of interneurons generating the extensor bursts, and vice versa (Figure 37-4B). This organizational feature is consistent with Graham Brown's notion that mutually inhibiting half-centers produce the alternating burst activity in flexor and extensor motor neurons. The interneurons mediating the reflexes from the flexor reflex afferents have not yet been fully identified, but they may include interneurons in the intermediate region of the gray matter in the sixth lumbar segment. Interneurons in this region of the cord produce prolonged bursts of activity in response to brief stimuli to either ipsilateral or contralateral FRA in spinal cats treated with L-DOPA (Figure 37-4C).
- Figure 37-5 The lamprey swims by means of a wave of muscle contractions traveling down one side of the body 180° out-of-phase with a similar traveling wave on the opposite side. This pattern can be detected by recording from different sites along the animal during normal swimming. Electromyogram recordings from four sites on the intact body are shown in the middle. The central origin of this pattern is revealed when a similar pattern is recorded from four spinal roots in an isolated cord (bottom). (Adapted from Grillner et al. 1987.) Figure 37-6 Some of the main features of the neuronal network in each body segment of the lamprey responsible for the rhythmic locomotor pattern for swimming. Activity in each segmental network is initiated by activity in glutaminergic axons descending from the reticular formation. The reticulospinal neurons increase the excitability of all neurons in the segmental networks by activation of both NMDA and non-NMDA receptors. On each side of the network excitatory interneurons (E) drive the motor neurons (MN) and two classes of inhibitory interneurons (I and L). The axons of the I interneurons cross the midline and inhibit all neurons in the contralateral half of the network, ensuring that when muscles on one side of the network are active, muscles on the other side are silent. The L interneurons inhibit the I interneurons on the same side. (Adapted from Grillner et al. 1995.) One of the best-analyzed GPGs is the one for lamprey swimming. Lampreys swim by alternating activation of motor neurons on the two sides of each body segment (Figure 37-5). Each segment contains a network capable of generating the rhythmic, alternating activity in motor neurons on the two sides (Figure 37-6). From these observations it is clear that the spinal pattern-generating network can generate a variety of motor patterns. Which pattern is generated depends on multiple factors, such as the supraspinal and tonic sensory inputs to the spinal pattern generators as well as the drugs used to initiate rhythmicity. This functional flexibility in the spinal pattern generator may be explained by a scheme in which mutually inhibiting half-centers produce the basic rhythmicity and establish a general pattern of reciprocity in the activity of flexor and extensor motor neurons while the details of the temporal pattern are established by the organization and properties of an interneuronal network between the half-centers and the motor neurons (Figure 37-7D). A number of cellular and synaptic mechanisms are involved in the initiation and termination of activity on one side of the network. One important mechanism in the initiation of activity is the opening of NMDA receptor-channels to produce plateau potentials. Once the inhibition from the contralateral I interneuron is terminated, NMDA receptor-channels in all ipsilateral neurons are opened by a depolarization resulting from postinhibitory rebound. The voltage-dependency of the NMDA receptor-channels then leads to the generation of plateau potentials. Activation of low-voltage Ca2+ channels further strengthens the depolarization. The influx of Ca2+ through these channels and the NMDA receptor-channels activates calcium-dependent K+ channels. The resultant increase in K+ conductance terminates the plateau potentials and so contributes to the termination of activity. Two additional mechanisms contribute to the termination of activity in each half of the network. One is a progressive decline in the discharge rate of the neurons resulting from the summation of slow after-hyperpolarizations. The other is delayed excitation of the L interneurons, which inhibit the I interneurons (Figure 37-6) and thereby remove inhibition from the contralateral half of the network, enabling it to become active.
- Figure 37-8 Information on hip extension controls the transition from stance to swing. A. Oscillating movements around the hip joint in an immobilized decerebrate cat entrains the fictive locomotor pattern in extensor and flexor motor neurons. Note that the flexor bursts, corresponding to the swing phase, are generated when the hip is extended. (Adapted from Kriellaars et al. 1994.) B. Stretching the detached hip flexor muscle (iliopsoas) in a walking decerebrate cat causes inhibition of extensor activity and an earlier onset of flexor activity. The arrow indicates the expected time of the onset of flexor activity had the flexor muscle not been stretched. Activation of sensory fibers from muscle spindles is responsible for this effect. (Adapted from Hiebert et al. 1996.) Sensory Input From Moving Limbs Regulates Stepping Patterns Although normal walking is automatic, it is not necessarily stereotyped. Outside the laboratory mammals constantly use sensory input to adjust their stepping patterns to variations in the terrain and to unexpected events. Three important types of sensory information are used to regulate stepping: somatosensory input from the receptors of muscle and skin, input from the vestibular apparatus (for controlling balance), and visual input. In considering the role of somatosensory input in stepping, Charles Sherrington made the distinction between proprioceptors and exteroceptors. Proprioceptors are located in muscles and joints and are excited by body movements; input from proprioceptors is involved in the automatic regulation of stepping. Exteroceptors are located in the skin; their main function is to adjust stepping to external stimuli. This distinction is still considered valid, although it is now recognized that skin afferents can provide important feedback about body movements.
- Figure 37-10 Descending signals from the brain stem and motor cortex initiate locomotion and adjust stepping movements to the immediate needs of the animal. The spinal locomotor system is activated by signals from the mesencephalic locomotor region (MLR) relayed via neurons in the medial reticular formation (MRF) (see Figure 37-11). The cerebellum receives signals via spinocerebellar pathways from both peripheral receptors and the spinal central pattern generators and adjusts the locomotor pattern via brain stem nuclei. Modification of stepping by visual signals is mediated via the motor cortex. Sensory Input From the Skin Allows Stepping to Adjust to Unexpected Obstacles Exteroceptors in the skin have a powerful influence on the central pattern generator for walking. One important function for these receptors is to detect external obstacles and adjust the stepping movements to avoid them. A well-studied example is the stumbling-corrective reaction in cats. A mild mechanical stimulus applied to the dorsal part of the paw during the swing phase produces excitation of flexor motor neurons and inhibition of extensor motor neurons, leading to rapid flexion of the paw away from the stimulus and elevation of the leg in an attempt to step over the object. Because this corrective response is readily observed in spinal cats, it must be produced to a large extent by circuits entirely contained within the spinal cord. One of the interesting features of the stumbling-corrective reaction is that corrective flexion movements are produced only when the paw is stimulated during the swing phase. An identical stimulus applied during the stance phase produces an opposite response: excitation of extensor muscles that reinforces the ongoing extensor activity. This extensor action is appropriate; if a flexion reflex were produced, the animal might collapse because its weight is being supported by the limb. This is an example of a phase-dependent reflex reversal: the same stimulus will excite one group of motor neurons during one phase of locomotion and excite the antagonist motor neurons during another phase. Descending Pathways Are Necessary for Initiation and Adaptive Control of Walking Although the basic motor pattern for stepping is generated in the spinal cord, fine control of stepping movements involves numerous regions of the brain, including the motor cortex, cerebellum, and various sites within the brain stem. Recordings from neurons in all these regions have shown that many are rhythmically active during locomotor activity and hence involved in some way with the production of the normal motor pattern. Each region, however, appears to play a different role in the regulation of locomotor function. Supraspinal regulation of stepping can, in broad terms, be divided into three functional systems. One activates the spinal locomotor system and controls the overall speed of locomotion, another refines the motor pattern in response to feedback from the limbs, and the third guides limb movement in response to visual input (Figure 37-10).
- Figure 37-11 Locomotor responses to electrical stimulation of the mesencephalic locomotor region. A. Increasing the strength of stimulation to the mesencephalic locomotor region (MLR) in a decerebrate cat walking on a treadmill progressively changes the gait and rate of stepping from slow walking to trotting and finally to galloping. As the cat progresses from trotting to galloping the hind limbs shift from alternating to simultaneous flexion and extension. B. Efferent projection from the MLR. Stimulation of the MLR excites interneurons in the medial reticular formation whose axons descend to the spinal locomotor system via the ventrolateral funiculus (VLF). (Adapted from Mori et al. 1992.) Descending Pathways From the Brain Stem Initiate Walking and Control Its Speed In their seminal studies of decerebrate cats, Mark Shik, Fidor Severin, and Grigori Orlovsky found that tonic electrical stimulation of the mesencephalic locomotor region initiates stepping when animals are placed on a freely moving treadmill. The rhythm of the locomotor pattern is not related to the pattern of electrical stimulation but depends only on its intensity. Weak stimulation produces a walking gait that increases in speed as the intensity increases; progressively stronger stimulation produces trotting and finally galloping (Figure 37-11A). Thus, a relatively simple control signal from the brain stem, modulated only in intensity, not only initiates locomotion but also controls the overall speed of walking. It is especially interesting to note that the changes in gait are associated with changes in the coordination between legs: an out-of-phase relationship between left and right legs in walking changes to an in-phase relationship in galloping. These shifts in interlimb coordination are most likely implemented by local circuits in the spinal cord, since they are also observed in spinal cats walking on a motorized treadmill as the treadmill speed is increased. In addition to the mesencephalic locomotor region several other motor regions of the brain, including a subthalamic motor region and a pontine locomotor region, can produce locomotion when stimulated experimentally. How these different brain stem regions interact in normal control of locomotion is not yet known. The Descending Signals That Initiate Locomotion Are Transmitted via the Reticulospinal Pathway How are signals from brain stem locomotor regions transmitted to pattern-generating networks in the spinal cord? Because application of adrenergic drugs is often sufficient to initiate stepping in acute spinal animals, an early hypothesis was that the initiation and maintenance of locomotor activity depends on activity in the descending noradrenergic pathway from the locus ceruleus or the descending serotonergic pathway from the raphe nucleus. However, neither of these two aminergic pathways is essential for locomotion, since locomotor activity can be evoked after depletion of these amines. The current view is that biogenic amines regulate the magnitude and timing of motor neuron activity in the locomotor networks in the spinal cord. Thus, while adrenergic drugs can initiate stepping movements in the spinal preparation, aminergic systems may not serve this function in intact animals. If aminergic systems do not initiate locomotion, what descending signals are necessary for the initiation of locomotor activity? Clues to the answer to this question have come from studies on the initiation of locomotor activity in the neonatal rat and lamprey. In both animals administration of glutamate receptor agonists initiates locomotor activity. Recently a similar phenomenon has been observed in the cat. In decerebrate cats intrathecal administration of agonists that bind to NMDA-type glutamate receptors in the spinal cord initiates locomotion, while application of glutamate receptor antagonists prevents initiation of locomotor activity when the mesencephalic locomotor region is stimulated. These observations suggest that descending glutaminergic pathways are involved in initiating locomotor activity. Considerable research has focused on identifying the origin and location of the pathways involved in initiating locomotor activity. The axons of neurons in the nuclei near the mesencephalic locomotor region do not descend directly to the cord and therefore do not directly activate central pattern generators. Instead, neurons in the medullary reticular formation, whose axons descend in the ventrolateral region of the spinal cord, are considered important elements in initiating locomotor activity (Figure 37-11B). These neurons are excited by stimulation of the mesencephalic locomotor region, and transection of their axons in the ventrolateral funiculus of the spinal cord prevents stimulation of the mesencephalic locomotor region from initiating locomotor activity. Thus, the current evidence indicates that the signals that activate locomotion and control its speed are transmitted to the spinal cord by glutaminergic neurons whose axons travel in the reticulospinal pathway.
- Figure 37-12 Activity in neurons in the motor cortex is modulated by visual system inputs to adapt stepping movements. When a normal cat steps over objects fixed to the belt of a treadmill, neurons in the motor cortex increase in activity. This increase in cortical activity is associated with enhanced activity on electromyograms (EMGs) in foreleg muscles. (Adapted from Drew 1988.) The Motor Cortex Is Involved in the Control of Precise Stepping Movements in Visually Guided Walking During normal walking we often must guide our walking using visual cues. The motor cortex is essential in such visuomotor coordination. Experimental lesions of the motor cortex do not prevent animals from walking on a smooth floor or even on smooth inclines. However, they do severely impair tasks requiring a high degree of visuomotor coordination, such as walking on the rungs of horizontal ladders, stepping over a series of barriers, and stepping over single objects placed on a treadmill belt. Such “skilled walking” is associated with considerable modulation in the activity of a large number of neurons in the motor cortex (Figure 37-12). Since many of these neurons project directly into the spinal cord, they may regulate the activity of interneurons that form part of, or are influenced by, the central pattern generator for locomotion.
- The Cerebellum Fine-Tunes the Locomotor Pattern by Regulating the Timing and Intensity of Descending Signals Damage to the cerebellum results in marked abnormalities in locomotor movements, including abnormal variations in the speed and range of movements at different joints in single limbs and abnormal coupling between stepping in different limbs. These symptoms are collectively referred to as ataxia (see Chapter 42). Ataxic walking resembles a drunken gait. Since ataxic gait is apparent in patients with cerebellar lesions even when they are walking on a flat, smooth surface, we can conclude that the cerebellum is involved in the regulation of all stepping movements. The cerebellum receives information about both the actual stepping movements and the state of the spinal rhythm-generating network via two ascending pathways. For the hind legs of the cat these are the dorsal and ventral spinocerebellar tracts. Neurons in the dorsal tract are strongly activated by numerous leg proprioceptors and thus provide the cerebellum with detailed information about the biomechanical state of the hind legs. In contrast, neurons in the ventral tract are activated primarily by interneurons in the central pattern generator, thus providing the cerebellum with information about the state of the spinal locomotor network. It is thought that the cerebellum compares the actual movements of the legs (proprioceptive signals in the dorsal spinocerebellar tract) with the intended movements (information on the central pattern generator carried by the ventral spinocerebellar tract) and computes corrective signals that are then sent to various brain stem nuclei (see Figure 37-10). Thus the cerebellum may adjust the locomotor pattern when stepping movements unexpectedly deviate from the intended movements. The brain stem nuclei influenced by the cerebellum during walking include the vestibular nuclei, red nucleus, and nuclei in the medullary reticular formation. Cerebellar output to the vestibular nuclei may be involved in integrating proprioceptive information from the legs with vestibular signals for the control of balance. Human Walking May Involve Spinal Pattern Generators Unlike spinal cats and other quadrupeds, humans with spinal lesions that effectively transect the spinal cord generally are not able to walk spontaneously. Nevertheless, some observations of patients with spinal cord injury parallel the findings from studies of spinal cats. In one striking case, only recently reported, an individual with near complete transection of the spinal cord showed spontaneous uncontrollable rhythmic movements of the legs when the hips were extended. This closely parallels the finding that rhythmic stepping movements can often be evoked in chronic spinal cats by hip extension. In another study a drug influencing the biogenic amines (clonidine) was found to improve stepping on a treadmill in a few patients with severe spinal cord injury, as it does in spinal cats. Compelling evidence for the existence of spinal rhythm-generating networks in humans comes from studies of development. Human infants produce rhythmic stepping movements immediately after birth if held erect and moved over a horizontal surface. This strongly suggests that some of the basic neuronal circuits for locomotion are established genetically. These circuits must be located at or below the brain stem (possibly entirely within the spinal cord) since stepping can occur in anencephalic infants. These basic circuits are thought to be brought under supraspinal control in two ways during the first year of life, as automatic stepping is transformed into functional walking. First, the infant develops the ability to control locomotion voluntarily. From what we know about the neuronal mechanisms in the cat, this ability could depend on the development of reticulospinal pathways and regions activating reticulospinal neurons (such as the mesencephalic locomotor region). Second, the stepping pattern gradually develops from a primitive flexion-extension pattern that generates little effective propulsion to the complex mature pattern. Again, based on studies of cats, it is plausible that this adaptation is a result of maturation of descending systems originating from the motor cortex and brain stem nuclei modulated by the cerebellum. We can conclude, therefore, that human walking relies on the same general principles of neuronal organization as walking in other mammals: Intrinsic oscillatory networks are activated and modulated by other brain structures and by afferent input. Nevertheless, human locomotion differs from most animal locomotion in that it is bipedal, placing significantly greater demands on descending systems that control balance during walking. Indeed, some investigators believe that what allows the infant to begin to walk independently at the end of the first year is not necessarily maturation of the stepping pattern, but instead maturation of the system that enables successful balance control. Contrast this with horses, which can stand and walk within hours after birth. It is likely, therefore, that the spinal networks that contribute to human locomotion are more dependent on supraspinal centers than those in quadrupedal animals. This dependence may in part explain the relatively few observations of spontaneous stepping movements in humans with spinal cord injury. An Overall View Locomotion in mammals typically involves rhythmic movements of the body and one or more appendages. These movements depend on the precise regulation of the timing and the strength of contractions in numerous muscles. Centrally located neuronal circuits, known as central pattern generators, can generate the basic motor pattern for locomotion even without afferent feedback from peripheral receptors. Numerous central pattern generators have now been analyzed at the cellular level, and it is clear that a wide variety of cellular, synaptic, and network properties are involved in these local networks. Central pattern generators are extremely flexible. Their cellular and synaptic properties can be modified by chemical signals, and their functioning depends on how they are activated and the pattern of afferent input they receive. Contemporary research on mammalian locomotion dates from the 1960s, when two important experimental animal preparations were introduced. In the decerebrate animal stepping can be initiated by electrical stimulation of a site in the brain stem (the mesencephalic locomotor region). In the spinal preparation centrally generated locomotor activity can be evoked after the administration of L-DOPA and nialamide. Investigations using these preparations have confirmed and extended fundamental observations made near the turn of the century, namely that the basic rhythm for locomotion is generated centrally in spinal networks, that the transition from stance to swing is regulated by afferent signals from leg flexor and extensor muscles, and that descending signals from the brain regulate the intensity of locomotion and modify stepping movements according to the terrain on which the animal is walking. Recent studies of humans with spinal cord injury and normal infants indicate that many of the basic features of the neural control of human bipedal walking are similar to those in quadrupedal locomotion.
